Improved anti-CD19 CAR-T cells
A technology of lymphocytes and extracellular regions, applied in the field of biomedicine, can solve problems such as cytokine release syndrome and toxic reactions, and achieve the effect of safe anti-tumor activity and mild proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] Cell lines and basic experimental techniques used in the embodiments of the present invention are as follows:
[0113] Generation of lentivirus and transduction of human T lymphocytes
[0114] Replication-defective lentiviral vectors were generated and collected by centrifugation for transduction of human T lymphocytes. The following is a brief introduction to the production and collection of lentiviral vectors: 293T cells were placed on a cell culture dish with a bottom area of 150-cm2, and according to the instructions, Express-In (purchased from Open Biosystems / ThermoScientific, Waltham, MA) Viral transduction of 293T cells. Add 15 μg of lentiviral transgenic plasmid, 5 μg of pVSV-G (VSV glycoprotein expression plasmid), 10 μg of pCMVR8.74 plasmid (Gag / Pol / Tat / Rev expression plasmid) and 174 μl of Express- In (concentration is 1 μg / μl). The supernatants were collected at 24 hours and 48 hours, and centrifuged for 2 hours using an ultracentrifuge at 28,000 rpm (B...
Embodiment 2
[0121] Example 2 Construction of vectors co-expressing non-functional EGFR and anti-CD19 chimeric antigen receptor
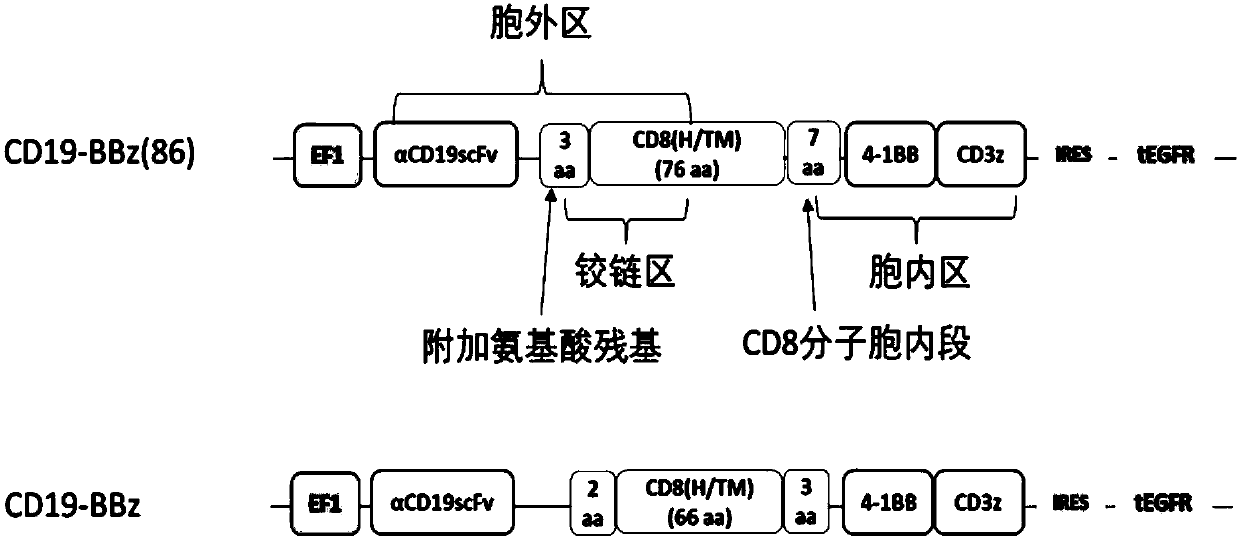
[0122] In this example, the inventors combined the XbaI and BstBI cloning site sequences at both ends and the sequence encoding the single-chain antibody against human CD19, human CD8α sequence, 4-1BB intracellular segment and T cell receptor combination ζ -Strand sequence was artificially synthesized, cloned into lentiviral vector shuttle plasmid containing EF-1 promoter (PCDH-EF1-MCS-IRES-GFP (System Biosciences, Palo Alto, CA). The inventor then artificially synthesized two ends connected with BspEI and SalI cloning site sequences and sequences encoding and expressing non-functional tEGFR, through double enzyme digestion, connection, screening and amplification of the target plasmid , to generate a lentiviral vector shuttle plasmid (named LV-CD19-BBz(86)) co-expressing anti-CD19 chimeric antigen receptor and non-functional tEGFR sequences.
[0123] figure 1...
Embodiment 3
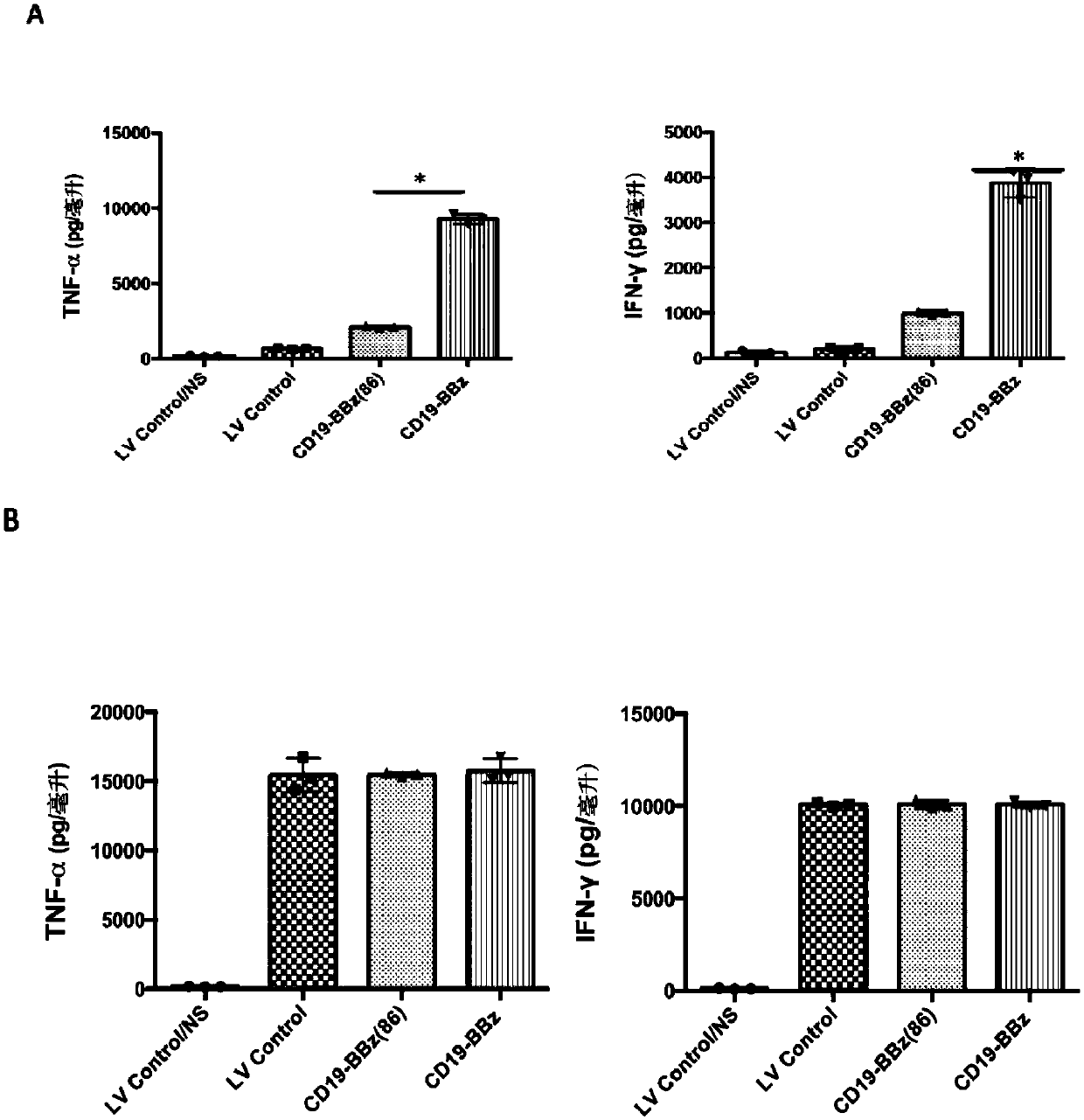
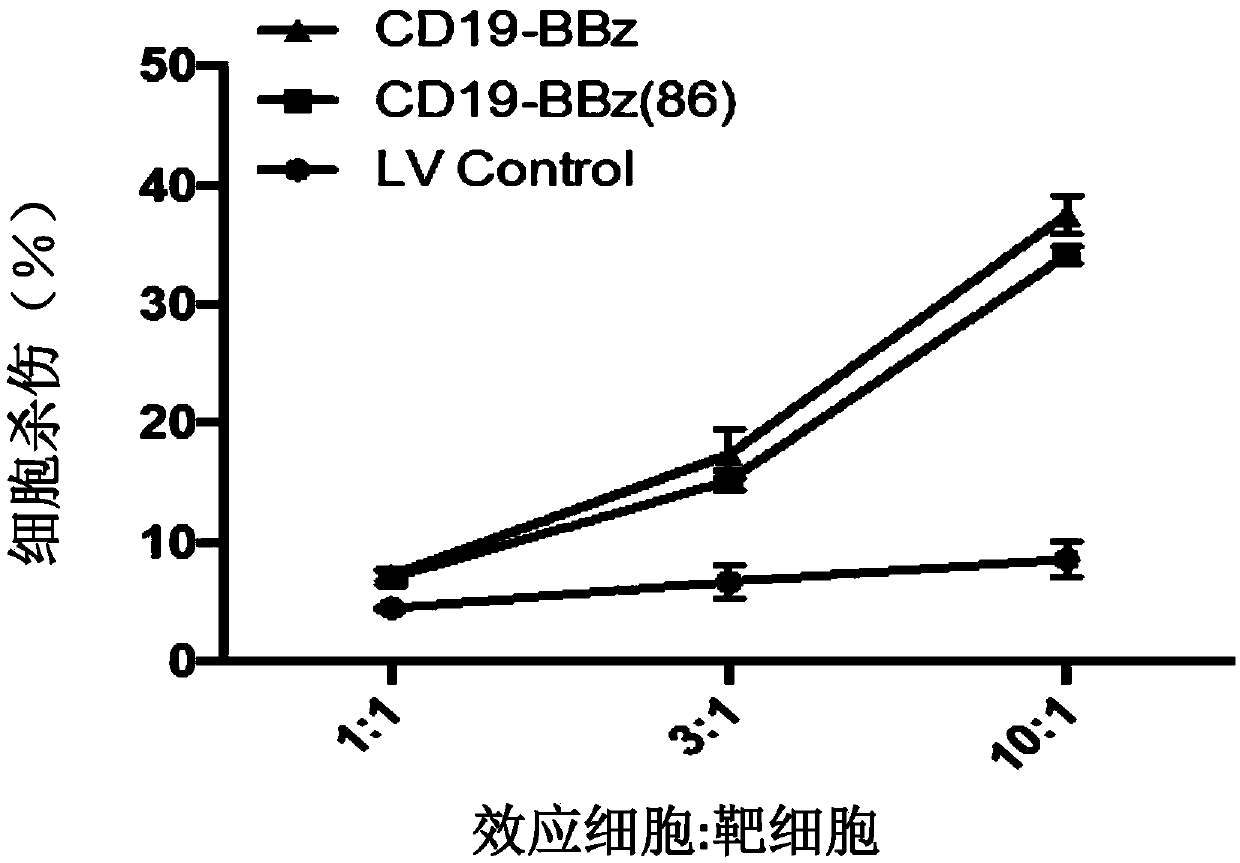
[0124] Example 3 The ability of improved LV-CD19-BBz(86) CAR-T lymphocytes to produce and secrete cytokines is significantly weakened
[0125]In this example, peripheral blood lymphocytes were obtained from anonymous blood donors. Peripheral blood lymphocytes were separated by gradient centrifugation using Ficoll-Hypaque. T lymphocytes and T cell activator magnetic beads CD3 / CD28 (purchased from Invitrogen, Carlsbad, CA) in 5% CO 2 , Incubated at 37 degrees Celsius for 72 hours, the medium was added with 2mmol / L glutamine, 10% high temperature inactivated fetal calf serum (FCS) (purchased from Sigma-Aldrich Co.) and 100U / ml of penicillin / chain RPMI medium 1640 (purchased from Invitrogen Gibco Cat. no. 12633-012) with antimycin double antibody. After activating and culturing for 72 hours, the cells were rinsed with washing solution to wash away the magnetic beads. The T cells were planted on cell culture dishes covered with recombinant fibronectin fragments (FN ch-296; Retro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com