Preparation method and quality detecting method of high-purity phlorizin
A high-purity technology for phlorizin, which is applied in the field of preparation of high-purity phlorizin and its quality inspection, and can solve the problems of unreached purity and inability to meet high-purity phlorizin chemical reference substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
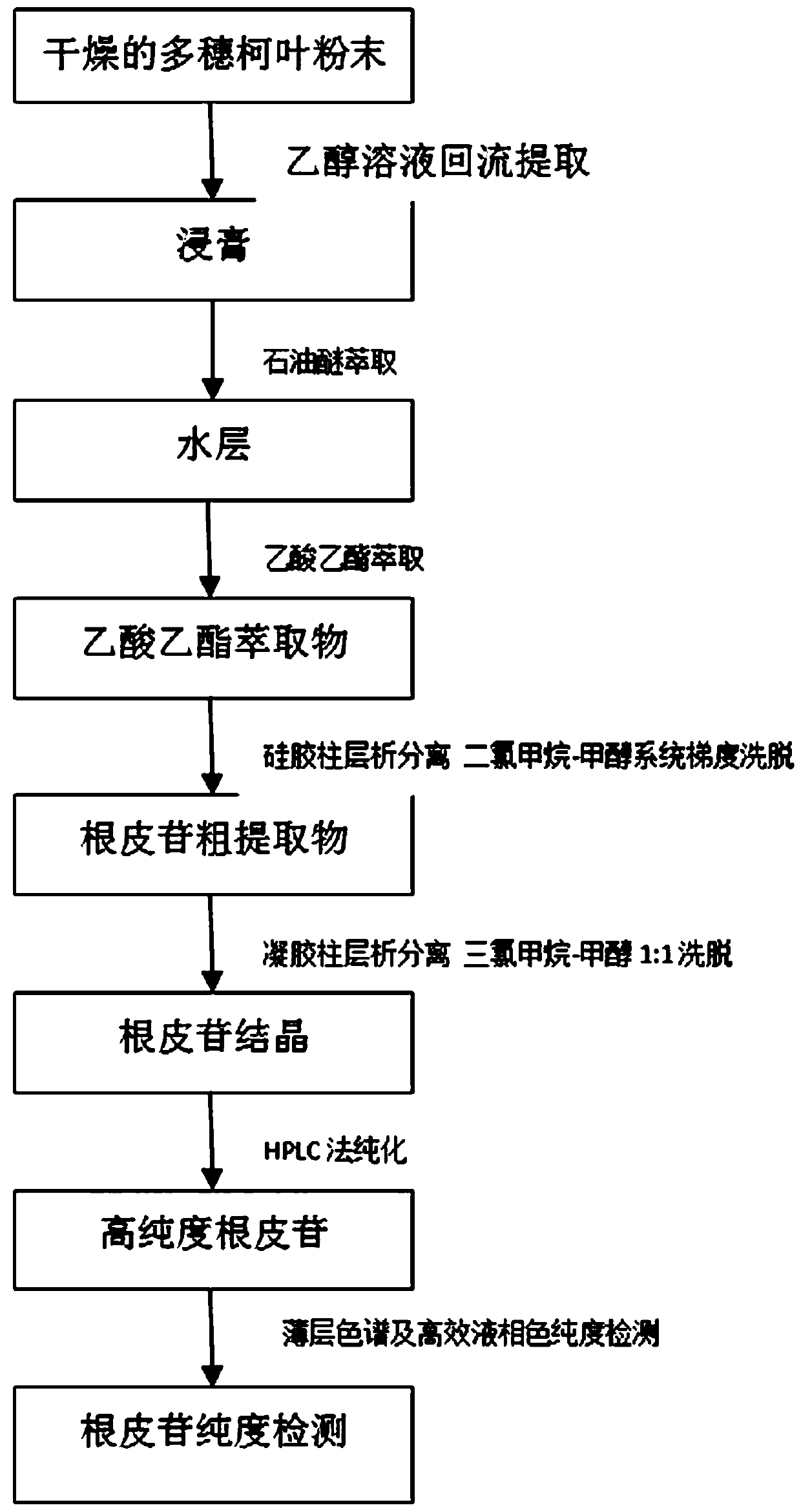
[0116] A preparation method for high-purity phlorizin, specifically comprising the steps of:
[0117] 1) 1 kg of dry leaf powder of Acaeaceae, reflux extraction with 10 times the weight of ethanol with a volume concentration of 70% for 3 times, filter the extract, combine the filtrates, recover the ethanol, and obtain the extract;
[0118] 2) Extract the extract with petroleum ether, let it stand, discard the petroleum ether layer, extract the water part with ethyl acetate, let it stand, combine the ethyl acetate layer, and concentrate to obtain the ethyl acetate extract;
[0119] 3) The ethyl acetate extract was chromatographed on a silica gel column and eluted with a methanol-dichloromethane system gradient to collect fractions containing phlorizin. The gradient elution conditions of the methanol-dichloromethane system were: 0- 5min, the elution system is dichloromethane; 5-15min, the elution system is methanol-dichloromethane, the volume ratio of methanol:dichloromethane is...
Embodiment 2
[0128] A preparation method for high-purity phlorizin, specifically comprising the steps of:
[0129] 1) 1 kg of dried leaf powder of Fructus Polycarpa, reflux extraction with 5 times the weight of ethanol with a volume concentration of 70% for 4 times, filter the extract, combine the filtrates, reclaim the ethanol, and obtain an extract;
[0130] 2) Extract the extract with petroleum ether, let it stand, discard the petroleum ether layer, extract the water part with ethyl acetate, let it stand, combine the ethyl acetate layer, and concentrate to obtain the ethyl acetate extract;
[0131] 3) The ethyl acetate extract was chromatographed on a silica gel column and eluted with a methanol-dichloromethane system gradient to collect fractions containing phlorizin. The gradient elution conditions of the methanol-dichloromethane system were: 0~ 8 minutes, the elution system is dichloromethane; 8-20min, the elution system is methanol-dichloromethane, the ratio of methanol:dichloromethan...
Embodiment 3
[0140] A preparation method for high-purity phlorizin, specifically comprising the steps of:
[0141] 1) 1 kg of dry leaf powder of Fructus Polycarpus is extracted 5 times with 50% ethanol at a volume concentration of 8 times the weight, the extract is filtered, the filtrate is combined, and the ethanol is reclaimed to obtain an extract;
[0142] 2) Extract the extract with petroleum ether, let it stand, discard the petroleum ether layer, extract the water part with ethyl acetate, let it stand, combine the ethyl acetate layer, and concentrate to obtain the ethyl acetate extract;
[0143] 3) The ethyl acetate extract was chromatographed on a silica gel column and eluted with a methanol-dichloromethane system gradient to collect fractions containing phlorizin. The gradient elution conditions of the methanol-dichloromethane system were: 0~ 5 minutes, the elution system is dichloromethane; 5-15min, the elution system is methanol-dichloromethane, the ratio is 1:100; 15-40min, the e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com