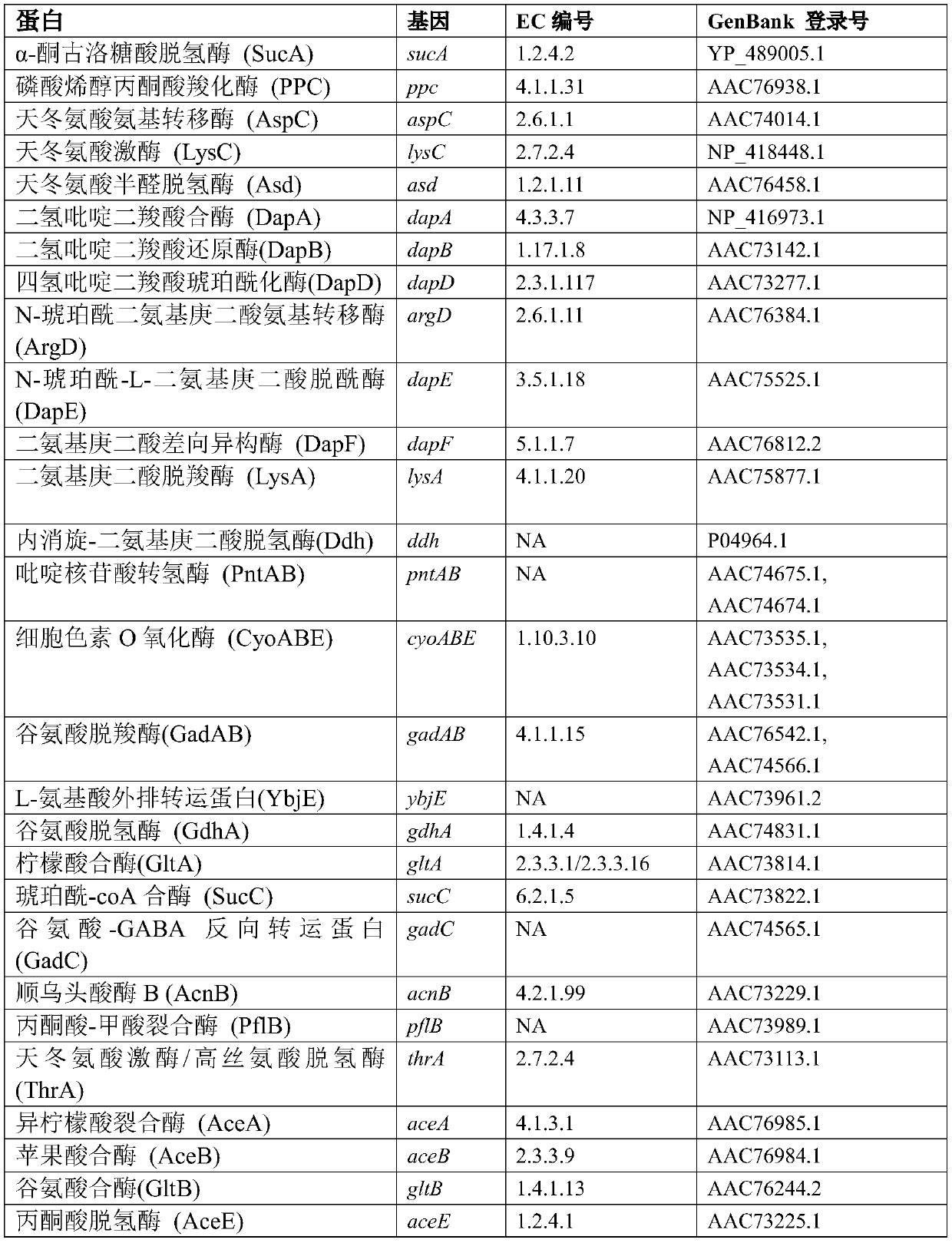
Modifications to lysine decarboxylase enzymes
A technology of lysine residues and amino acids, applied in the field of modification of lysine decarboxylase enzymes, can solve the problem that there is no literature describing CadA
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0067] Preparation of recombinant vector
[0068] The recombinant vector for expressing the variant CadA protein can be prepared using methods well known in the art. For example, the DNA sequence encoding the CadA variant polypeptide can be combined with transcription and other regulatory sequences that will direct the sequence from the gene in the desired cell (e.g., bacterial cells such as Hafnia alves, Escherichia coli, or glutamine). Corynebacterium acidi). In some embodiments, the expression vector comprising an expression cassette further comprises a promoter operably linked to the nucleic acid sequence encoding the CadA variant polypeptide, the expression cassette comprising a gene encoding the CadA variant polypeptide. In other embodiments, the promoter and / or other regulatory elements that direct the transcription of the cadA polynucleotide encoding the variant Cada polypeptide are endogenous to the host cell, and the expression of the cadA gene is introduced, for examp...
Embodiment 1
[0098] Example 1: Construction of a plasmid vector encoding CadA
[0099] A plasmid vector containing wild-type E. coli cadA (SEQ ID NO: 1) (which encodes the lysine decarboxylase CadA (SEQ ID NO: 2)) uses PCR primers cadA-F and cadA-R ( figure 1 ) Amplify from Escherichia coli MG1655 K12 genomic DNA, digest the plasmid with restriction enzymes Sad and XbaI, and ligate into pUC18 to generate plasmid pCIB60. The PCR primers cadA-F2 and cadA-R2 were used to optimize the 5'sequence upstream of the cadA gene to generate pCIB71.
Embodiment 2
[0100] Example 2: Construction of a plasmid vector encoding CadA with a cysteine mutation at the predicted interface amino acid residue.
[0101] A primer pair was designed to introduce mutations into the CadA gene of pCIB71 to modify the amino acid at positions 287, 290, 319, 320, 325, 346, 357, 381, 477, or 500 of CadA to cysteine using Quickchange PCR. DNA sequencing was used to verify the mutation, and the plasmids carrying cysteine mutations were labeled pCIB71-K287C, pCIB71-K290C, pCIB71-K319C, pCIB71-K320C, pCIB71-K325C, pCIB71-K346C, pCIB71-K357C, pCIB71-K381C, pCIB71 -K477C or pCIB71-K500C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com