Phenylhydrazone fluorescent probe, preparation and applications thereof
A technology of fluorescent probes and phenylhydrazones, which is applied in the field of fluorescent chemical sensing, can solve the problems of less fluorescence performance and less fluorine-boron-fluorescence complexation of the phenylhydrazone structure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Into a 250 mL three-neck round bottom flask, put 80% hydrazine hydrate (31.25 g, 0.37 mol) and dimethyl carbonate (54 g, 0.6 mol). Heat up to 80°C and reflux for 7 hours. After the reaction was completed, methanol and excess dimethyl carbonate were distilled off under reduced pressure until the end of the distillation was reached. After a large amount of foam was generated, the distillation was stopped. After cooling to room temperature, a white solid was precipitated, and the product was recrystallized with ethanol to obtain 25.5 g of white crystal IV-1. Yield 74.91%, m.p. 72-73°C.
Embodiment 2
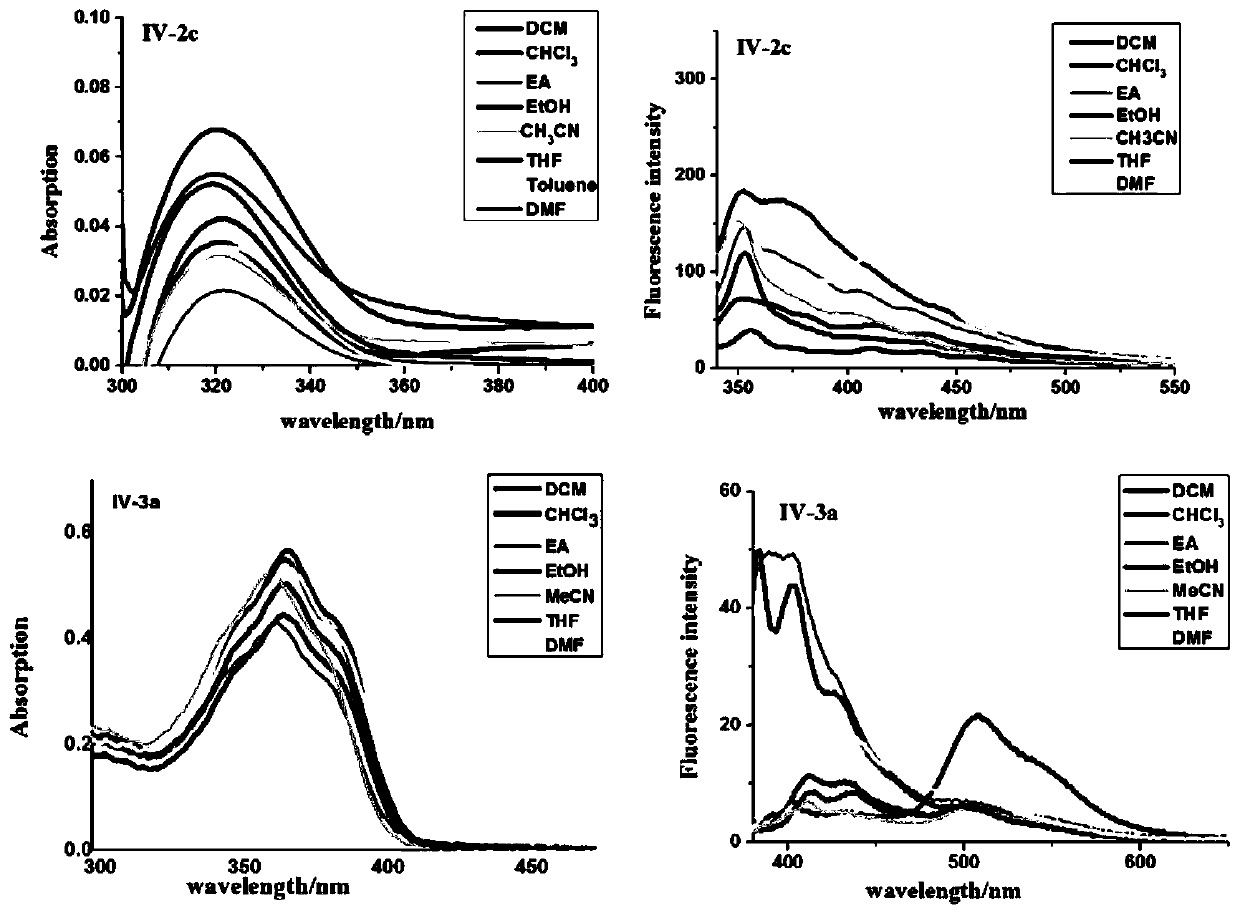
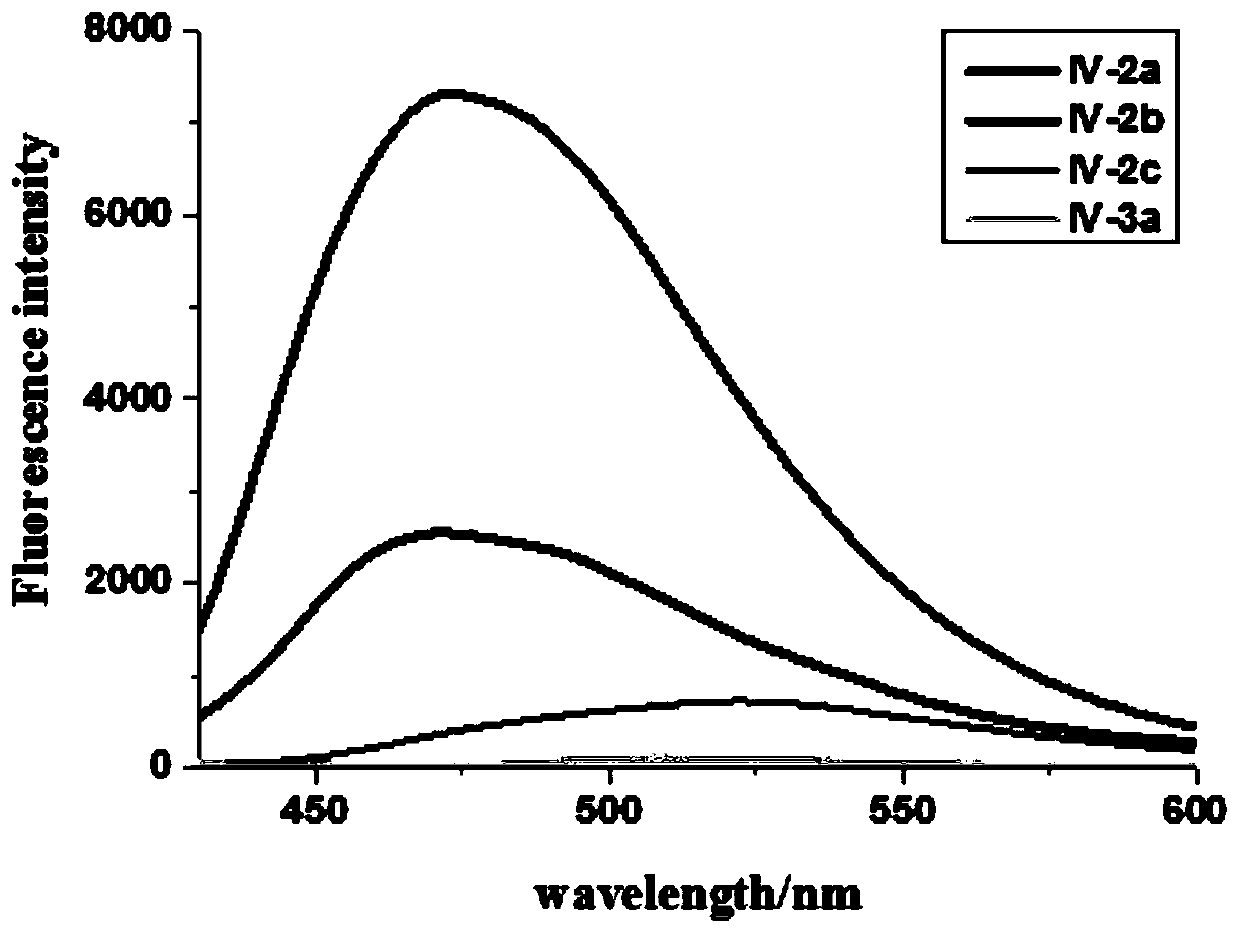
[0042] In a 50mL three-necked round-bottom flask, add 2,4-dihydroxyacetophenone (1.52g, 10mmol), methyl carbazate (0.92g, 10mmol), ethanol (25mL), and heat to reflux. TLC tracking detection (developer toluene: ethyl acetate = 9:1), after the reaction, cooled to room temperature, put into the refrigerator, precipitated a large amount of white solid, filtered with suction, rinsed with a small amount of ethanol, and recrystallized from ethanol to obtain a white powder IV-2a 1.95g, yield 87.1%, m.p.208.8°C-212.3°C. 1H NMR (500MHz, DMSO) δ: 13.04(s, 1H), 10.59(s, 1H), 9.72(s, 1H), 7.33(d, J=8.7Hz, 1H), 6.29(dd, J=8.7, 2.4Hz, 1H), 6.23(d, J=2.4Hz, 1H), 3.74(s, 3H), 2.23(s, 3H); HRMS(ESI) m / z calcd for: C 10 h 13 N 2 o 4 + (M+H) + 225.0869, found 225.0869.
Embodiment 3
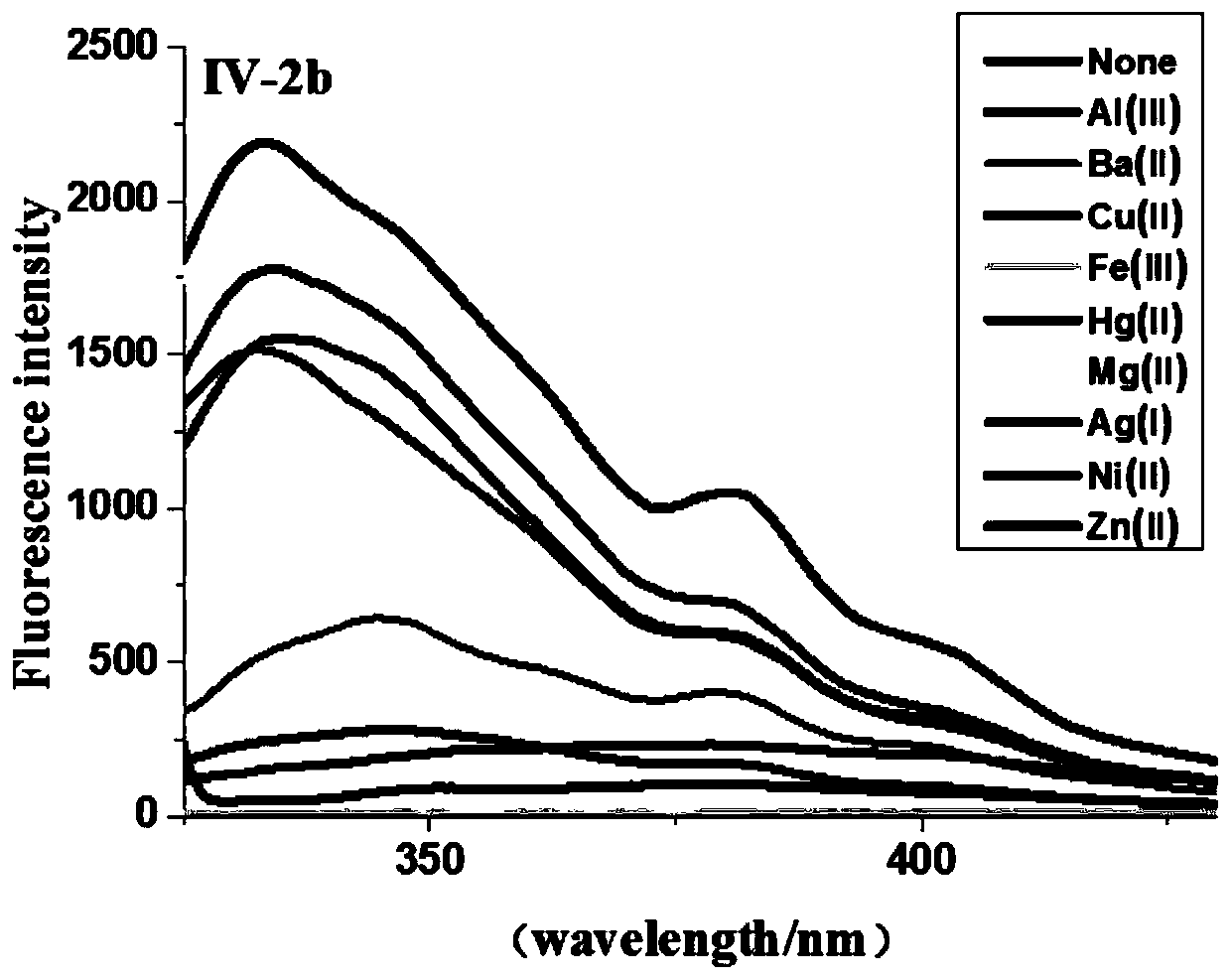
[0044] In a 50 mL three-neck round bottom flask, add 2,4-dihydroxybenzaldehyde (1.38 g, 10 mmol), methyl carbazate (0.92 g, 10 mmol), methanol (20 mL), and heat up to reflux. TLC tracking detection (developer toluene: ethyl acetate = 8:2), after the reaction, after cooling to room temperature, put it in the refrigerator, a large amount of white solid precipitated, filtered with suction, rinsed with a small amount of methanol, and recrystallized with ethanol to obtain a white solid. Crystal IV-2b 1.77g, yield 84.3%, m.p.109°C-113°C. 1 HNMR (500MHz, DMSO) δ: 11.07 (d, J = 42.9Hz, 2H), 9.81 (s, 1H), 8.10 (s, 1H), 7.20 (dd, J = 8.3, 2.8Hz, 1H), 6.44- 6.20(m,2H),3.69(s,3H);HRMS(ESI)m / z calcd for C 9 h 11 N 2 o 4 + (M+H) + 211.0713, found 211.0713.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com