Pharmaceutical composition for preventing or treating aging-related diseases containing decursin derivative as active ingredient
A technology for disease prevention and active ingredients, which is applied in the field of pharmaceutical compositions for the prevention or treatment of aging-related diseases containing a presenilin derivative as an active ingredient, can solve the problems of side effects, hindering the farnesylation of progerin, and the like, Achieve the effect of increasing collagen production, excellent presenilin expression and
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment
[0053] Hereinafter, to facilitate understanding, the following examples will be used in detail. However, the following examples are only for illustrating the content of the present invention, and the scope of the present invention is not limited by these examples. The embodiments of the present invention are provided to enable those skilled in the art to understand the present invention more completely.
[0054] Reference example: materials and equipment
[0055] 1 H and 13 The C NMR spectrum was measured using a JNM-AL 400 spectrometer (400MHz, JEOL, Japan), the melting point (Meltingpoint) was measured using an Electrothermal melting point apparatus (Yamaco.MD-S3), and the mass analysis instrument used an API 2000LC / MS / MS spectrometer (PE Sciex, Canada).
[0056] In addition, the optical rotations (Optical rotations) were measured by JASCO DIP-360 automatic digital polarimeter, and the purity of chiral substances was measured by HPLC (Shinadzu LC-6AD, Japan), chromatogr...
Embodiment 1
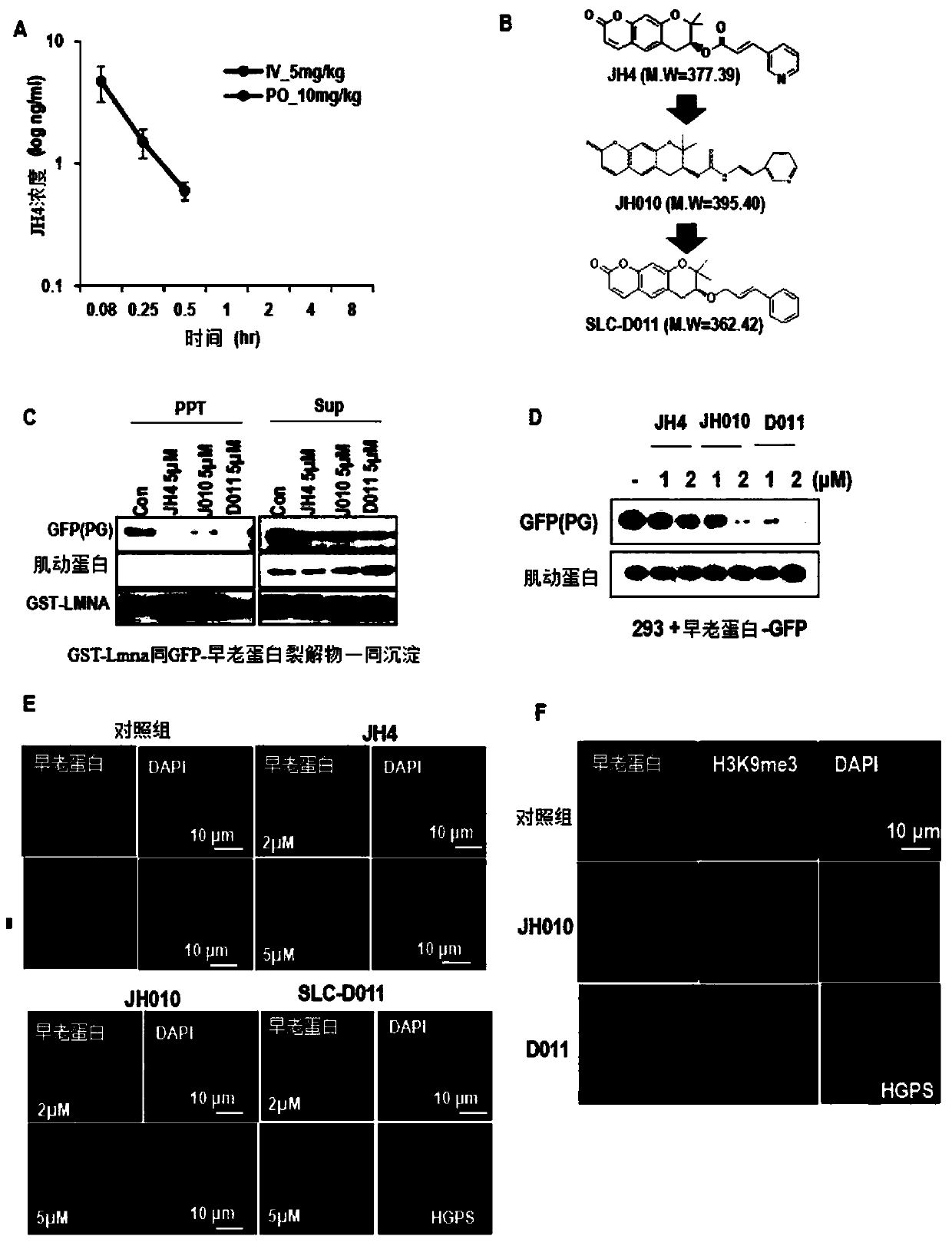
[0059]Example 1: Synthesis of (+)-Decursin Derivatives of Ether-form (SLC-D011)
[0060] (7S)-(+)-8,8-dimethyl-7-(3-phenyl-allyloxy)-7,8-dihydro-6H was synthesized according to the same process of the following reaction formulas 1 and 2 -pyrano[3,2-g]chromene-2-one ((7S)-(+)-8,8-dimethyl-7-(3-phenyl-allyoxy)-7,8-dihydro-6H- pyrano[3,2-g]chromen-2-one (SLC-D011)).
[0061] 1. Synthesis process I
[0062] Reaction 1
[0063]
[0064] Step I: add trans-cinnamic acid (trans-cinnamic acid, D011a, 5g, 33.7mmol) in the 100ml round bottom flask after dissolving with methanol (50ml), drop 5 drops of concentrated H 2 SO 4 , heated to reflux at 80°C for 24 hours, the reaction mixture was cooled to room temperature and then concentrated under reduced pressure.
[0065] Thereafter, liquid separation was performed with dichloromethane (300 ml) and distilled water (300 ml), and the organic layer was collected, dehydrated with sodium sulfate, and filtered.
[0066] The filtrate obtai...
Embodiment 2
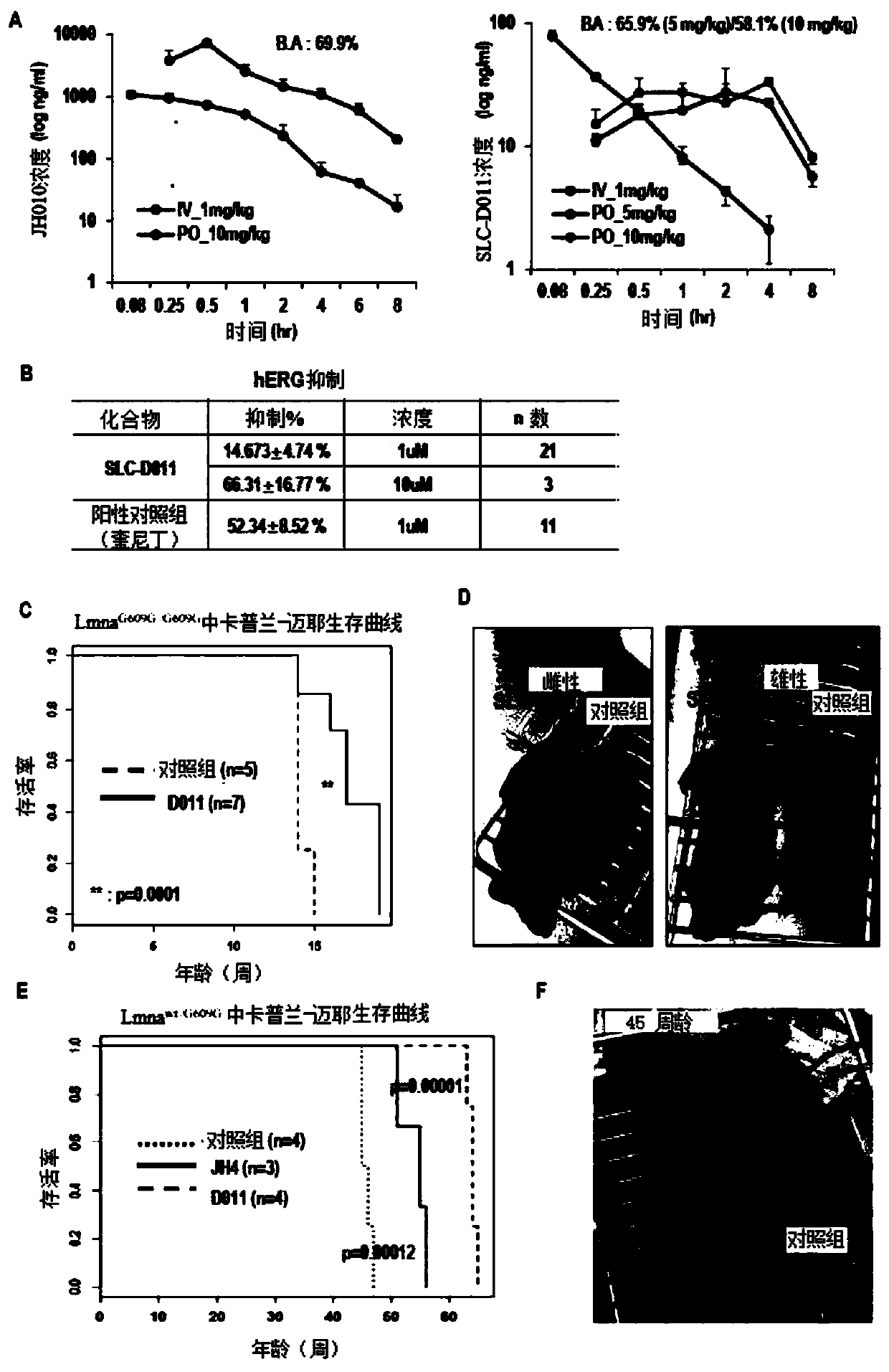
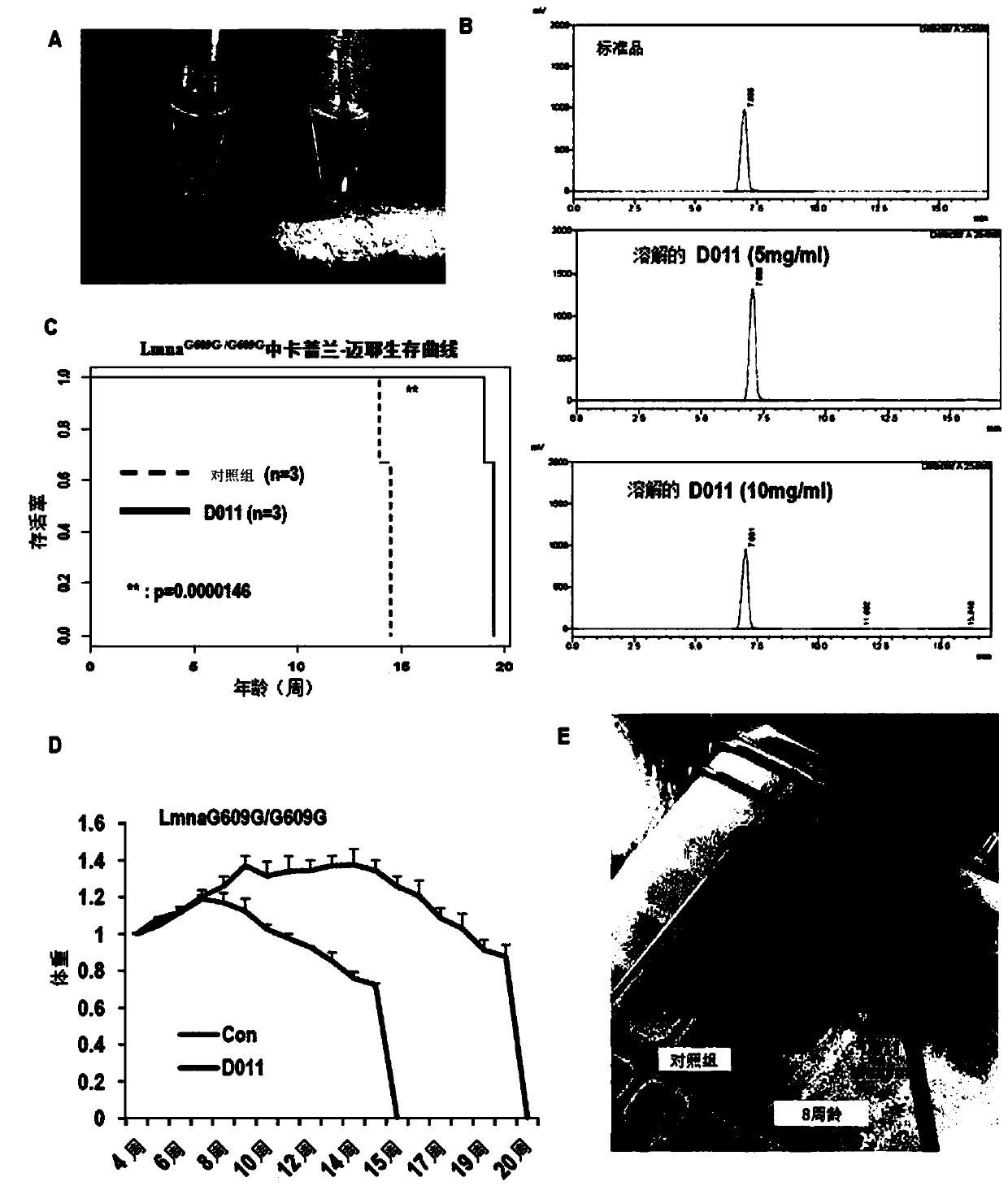
[0092] Example 2: Confirmation of the effect of SLC-D011 as a nuclear lamin A (LMNA)-presenilin binding inhibitor
[0093] 1. Animal experiments
[0094] Animal experiments were carried out in accordance with the animal policy recognized by Pusan National University, in the certification evaluation association and laboratory animal management certification organization.
[0095] Heterozygous Lmna provided by Carlos Lopez-Otin (University of Oviedo, Oviedo, Asturias, Spain) + / G609G Proper mating of Lmna G609G / 609G .
[0096] SLC-D011 20 mg / kg mixed with DMOS and PBS was intraperitoneally injected twice a week into 5-week-old mice. In addition, SLC-D011 dissolved in an olein-based solution (olein-based solution) at a concentration of 10 mg / ml was orally administered to mice five times a week, and mice in the control group were given only A solution based on glyceryl oleate.
[0097] Lmna G609G / 609G Mice were dosed starting at 5 weeks of age and treated with fresh compoun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com