Salt of benzoylaminopyridine derivative and application thereof in medicines
A medicine and a technology for preparing medicine, applied in the field of crystal forms and pharmaceutical compositions containing the hydrochloride, capable of solving problems such as undisclosed crystal forms and crystal structures of undisclosed compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Example 1 Hydrochloride Crystal Form I
[0098] 1. Preparation of hydrochloride crystal form I
[0099] Compound 5-(4-cyclopropyl-1H-imidazol-1-yl)-N-(6-(6,7-dihydro-5H-pyrrolo[2,1-c][1,2 ,4] Triazol-3-yl)pyridin-2-yl)-2-fluoro-4-methylbenzamide (765mg, 1.72mmol) was added to ethanol (14.0mL), beaten for 45 minutes, then added dropwise The self-made 1mol / L hydrochloric acid solution (3.4mL, 3.4mmol) was stirred for about 1 hour, then ethyl acetate (14.0mL) was added, stirred and crystallized for about 6 hours; suction filtered, and the filter cake was vacuum-dried at 60°C overnight to obtain a white Solid (527.3 mg, 59.3%).
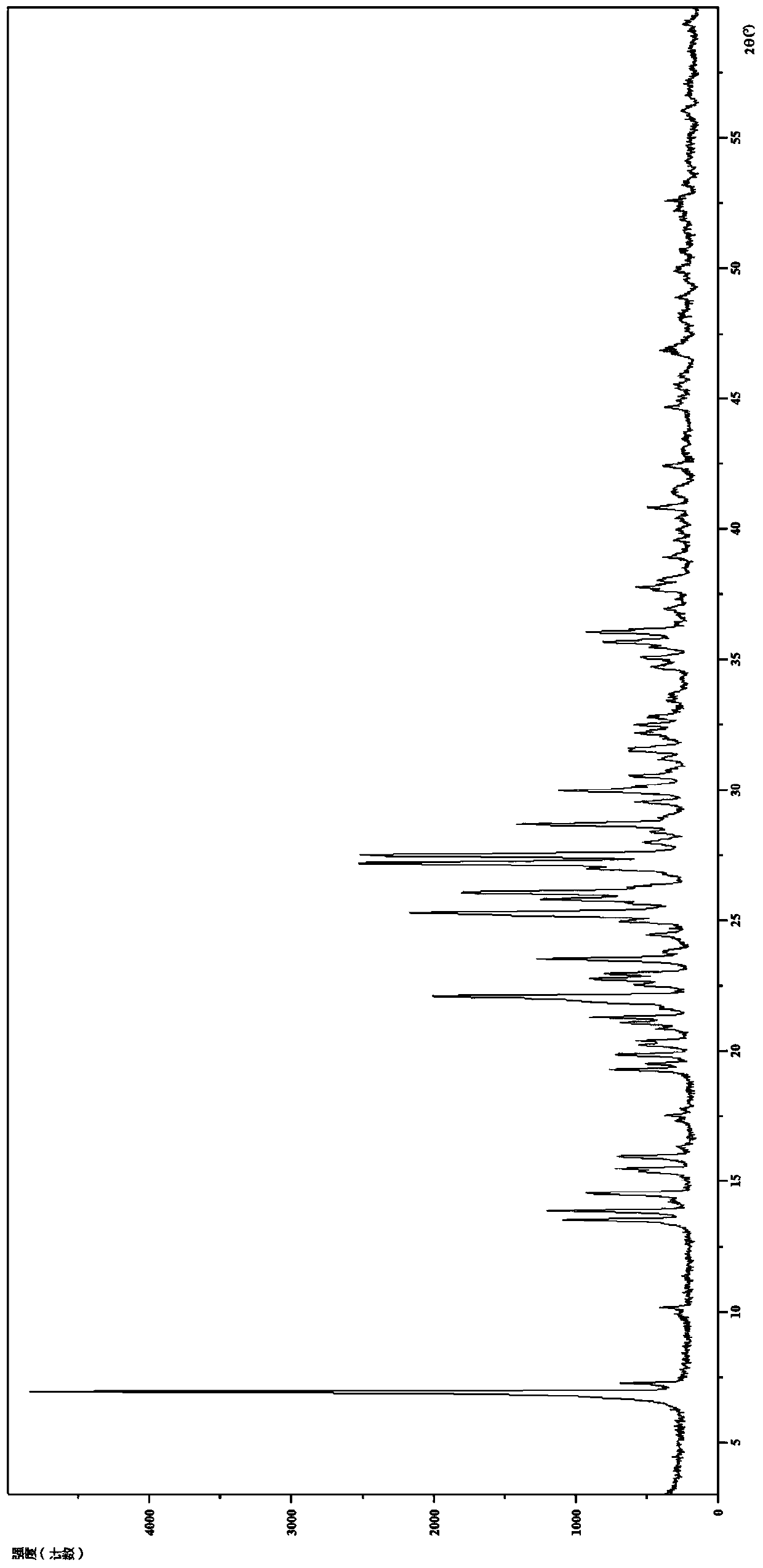
[0100] 2. Identification of Hydrochloride Form I
[0101](1) Analysis and identification by Empyrean X-ray powder diffraction (XRPD): using Cu-Kα radiation, it has the following characteristic peaks expressed in angle 2θ: 6.90°, 7.24°, 9.88°, 10.14°, 13.48°, 13.83°, 14.22 °,14.49°,15.41°,15.92°,16.30°,17.30°,17.51°,17.75°,19.25°,19.48°,19.8...
Embodiment 2
[0103] Example 2 Hydrochloride Form II
[0104] 1. Preparation of Hydrochloride Form II
[0105] The compound 5-(4-cyclopropyl-1H-imidazol-1-yl)-N-(6-(6,7-dihydro-5H-pyrrolo[2,1-c][1,2,4 ]triazol-3-yl)pyridin-2-yl)-2-fluoro-4-methylbenzamide (50.6mg, 0.114mmol) was added in isopropanol (1.0mL), and beating at 65°C for 30 minutes, Then dropwise added a self-made 1mol / L hydrochloric acid solution (0.13mL, 0.13mmol), incubated for 30 minutes, cooled naturally to room temperature, stirred and crystallized for about 9 hours; suction filtered, and the filter cake was vacuum-dried overnight at 60°C to obtain a white solid ( 44.2 mg, 80.7%).
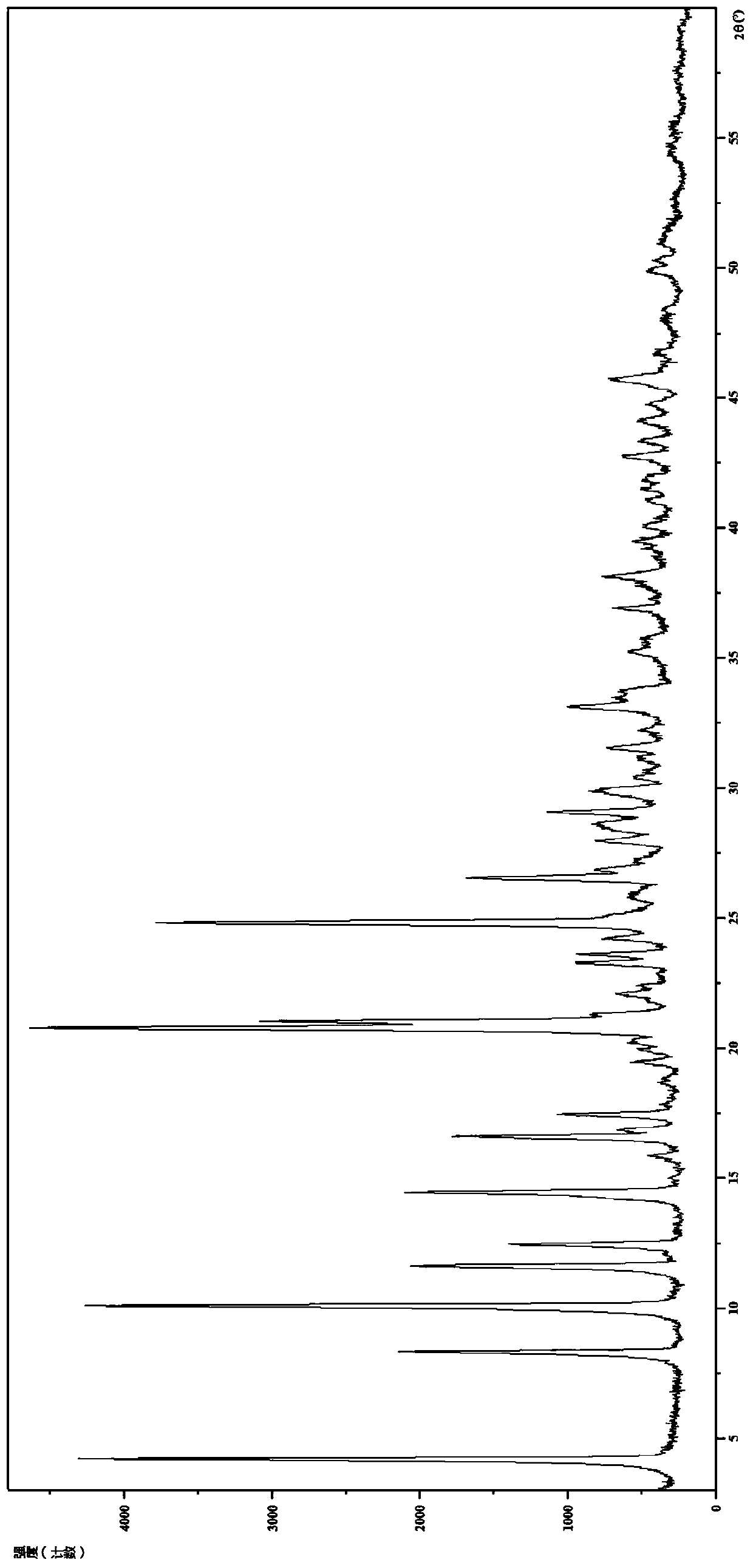
[0106] 2. Identification of Hydrochloride Form II
[0107] (1) Analysis and identification by Empyrean X-ray powder diffraction (XRPD): using Cu-Kα radiation, it has the following characteristic peaks expressed in angle 2θ: 4.18°, 8.28°, 10.04°, 11.57°, 12.41°, 14.39°, 15.83 °,16.57°,16.85°,17.42°,18.71°,19.43°,19.90°,20.20°,20.74°,21.00...
Embodiment 3
[0108] Example 3 Hydrochloride Form III
[0109] 1. Preparation of hydrochloride crystal form III
[0110] Compound 5-(4-cyclopropyl-1H-imidazol-1-yl)-N-(6-(6,7-dihydro-5H-pyrrolo[2,1-c][1,2 ,4] Triazol-3-yl)pyridin-2-yl)-2-fluoro-4-methylbenzamide (1005.4mg, 2.26mmol) was added to dimethyl sulfoxide (10.0ml) for beating for 1 hour , then dropwise added homemade 1mol / L hydrochloric acid solution (2.5mL, 2.5mmol), then added ethyl acetate (20.0mL) solution and stirred for 2 days; suction filtered, and the filter cake was vacuum-dried overnight at 80°C to obtain a white solid (368.5 mg, 33.9%).
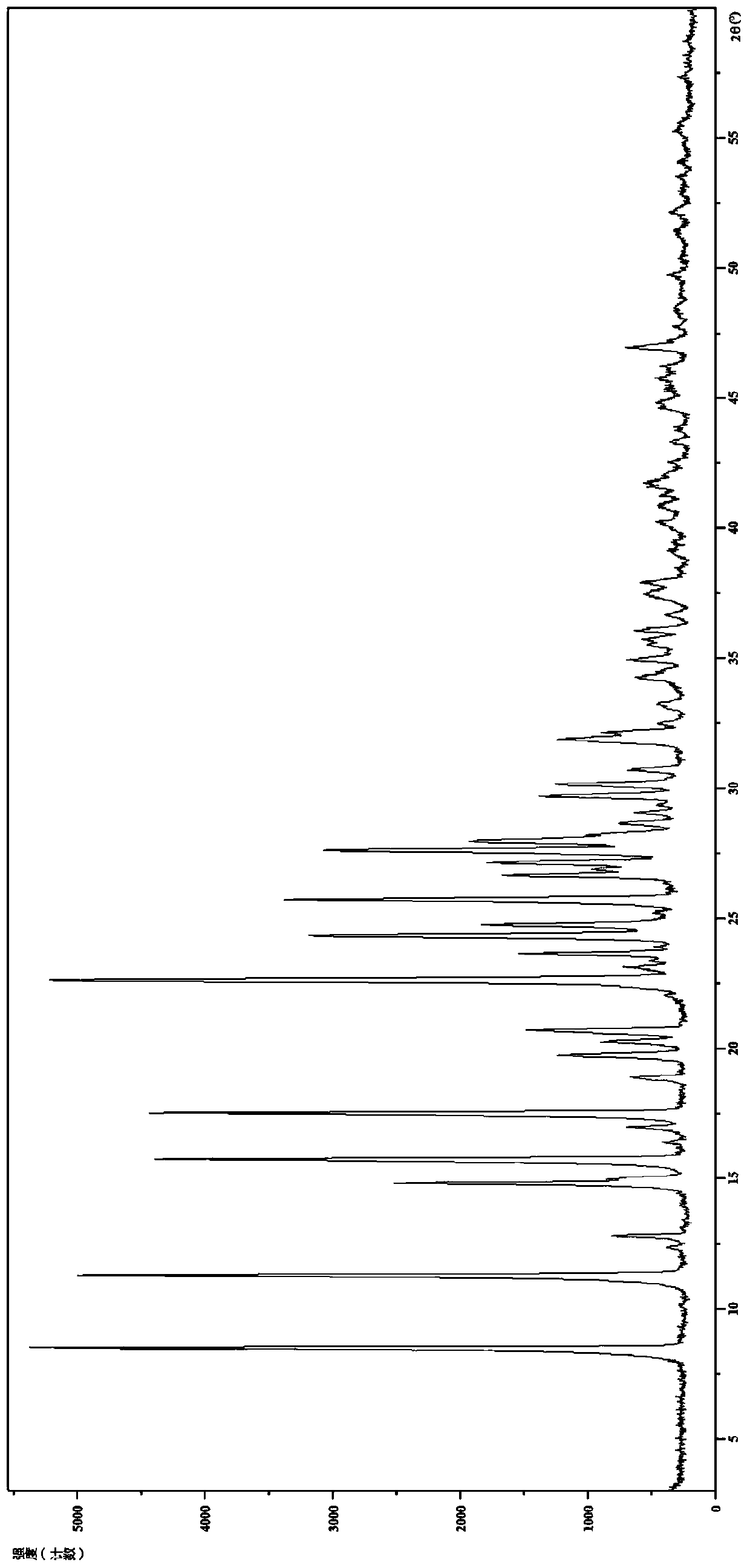
[0111] 2. Identification of Hydrochloride Form III
[0112] (1) Analysis and identification by Empyrean X-ray powder diffraction (XRPD): using Cu-Kα radiation, it has the following characteristic peaks expressed in angle 2θ: 8.45°, 11.24°, 12.32°, 12.76°, 14.79°, 14.99°, 15.70 °,16.37°,16.95°,17.48°,18.84°,19.71°,20.23°,20.64°,22.58°,23.09°,23.60°,24.29°,24.71°,25.66°,26.61°,26....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com