Salt of benzoylaminopyridine derivative and application thereof in medicines
A technology of diffraction peaks and compounds, applied in the field of medicine, can solve problems such as undisclosed and undisclosed crystal structures of compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
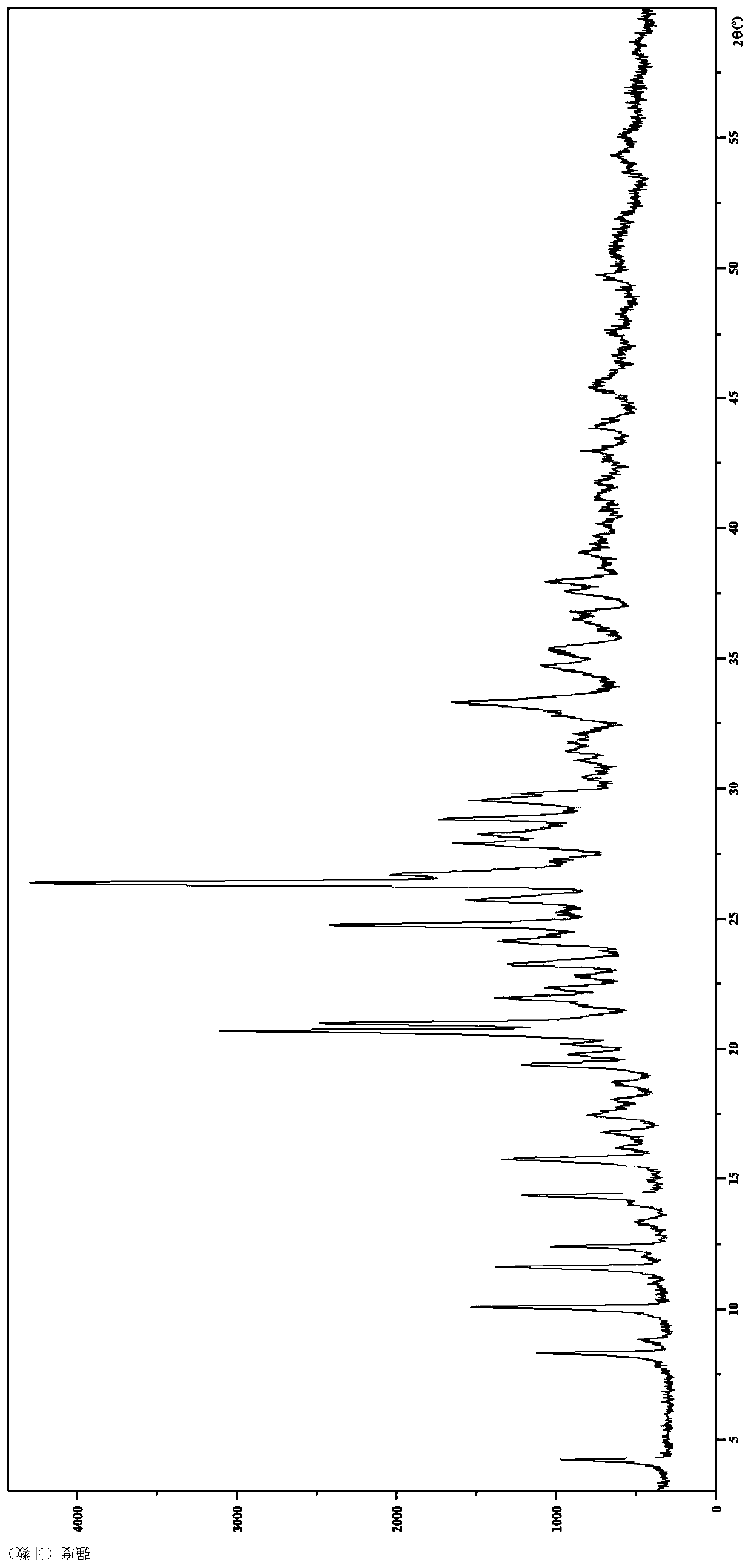
[0105] Embodiment 1 hydrobromide crystal form I
[0106] 1. Preparation of hydrobromide crystal form I
[0107] Compound 5-(4-cyclopropyl-1H-imidazol-1-yl)-N-(6-(6,7-dihydro-5H-pyrrolo[2,1-c][1,2 ,4] Triazol-3-yl)pyridin-2-yl)-2-fluoro-4-methylbenzamide (51.3mg, 0.116mmol) was added to isopropanol (1.0mL) and beaten for about 1 hour , and then dropwise added homemade 1mol / L hydrobromic acid solution (0.26mL, 0.26mmol), stirred for 10 hours and then suction filtered, and the filter cake was vacuum-dried overnight at 60°C to obtain a white solid (40.9mg, 67.4%).
[0108] 2. Identification of hydrobromide crystal form I
[0109] (1) Analysis and identification by Empyrean X-ray powder diffraction (XRPD): using Cu-Kα radiation, it has the following characteristic peaks expressed in angle 2θ: 4.18°, 8.26°, 8.77°, 10.03°, 11.57°, 12.37°, 13.30 °,14.03°,14.31°,15.73°,16.25°,16.73°,17.49°,18.02°,18.64°,19.36°,19.75°,20.15°,20.63°,20.96°,21.90°,22.32°,22.75°, 23.25°, 24.11°, 24...
Embodiment 2
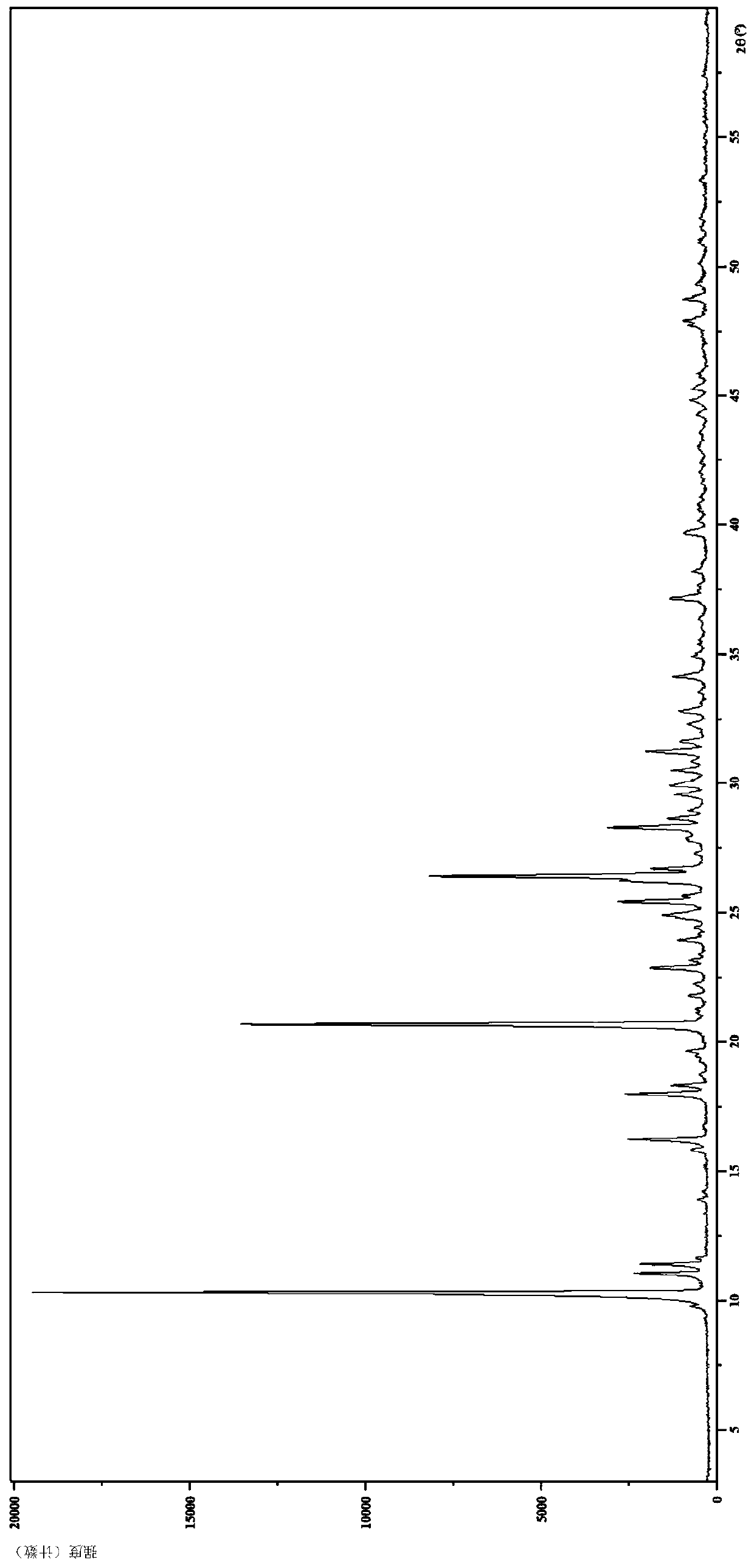
[0111] Embodiment 2 hydrobromide crystal form II
[0112] 1. Preparation of hydrobromide crystal form II
[0113] Compound 5-(4-cyclopropyl-1H-imidazol-1-yl)-N-(6-(6,7-dihydro-5H-pyrrolo[2,1-c][1,2 ,4] Triazol-3-yl)pyridin-2-yl)-2-fluoro-4-methylbenzamide (512.3mg, 1.155mmol) was added to acetone (10.0mL), beaten for 1 hour, then added Homemade 1 mol / L hydrobromic acid solution (1.3mL, 1.30mmol), stirred for 6 hours, suction filtered, and the filter cake was vacuum-dried at 60°C to obtain a white solid; the solid was beaten in ethanol (4.0mL) for about 10 hours After suction filtration, the filter cake was vacuum-dried overnight at room temperature to obtain a white solid (283.1 mg, 46.74%).
[0114] 2. Identification of hydrobromide crystal form II
[0115] (1) Analysis and identification by Empyrean X-ray powder diffraction (XRPD): using Cu-Kα radiation, it has the following characteristic peaks expressed in angle 2θ: 9.76°, 10.30°, 11.04°, 11.40°, 11.63°, 13.89°, 14....
Embodiment 3
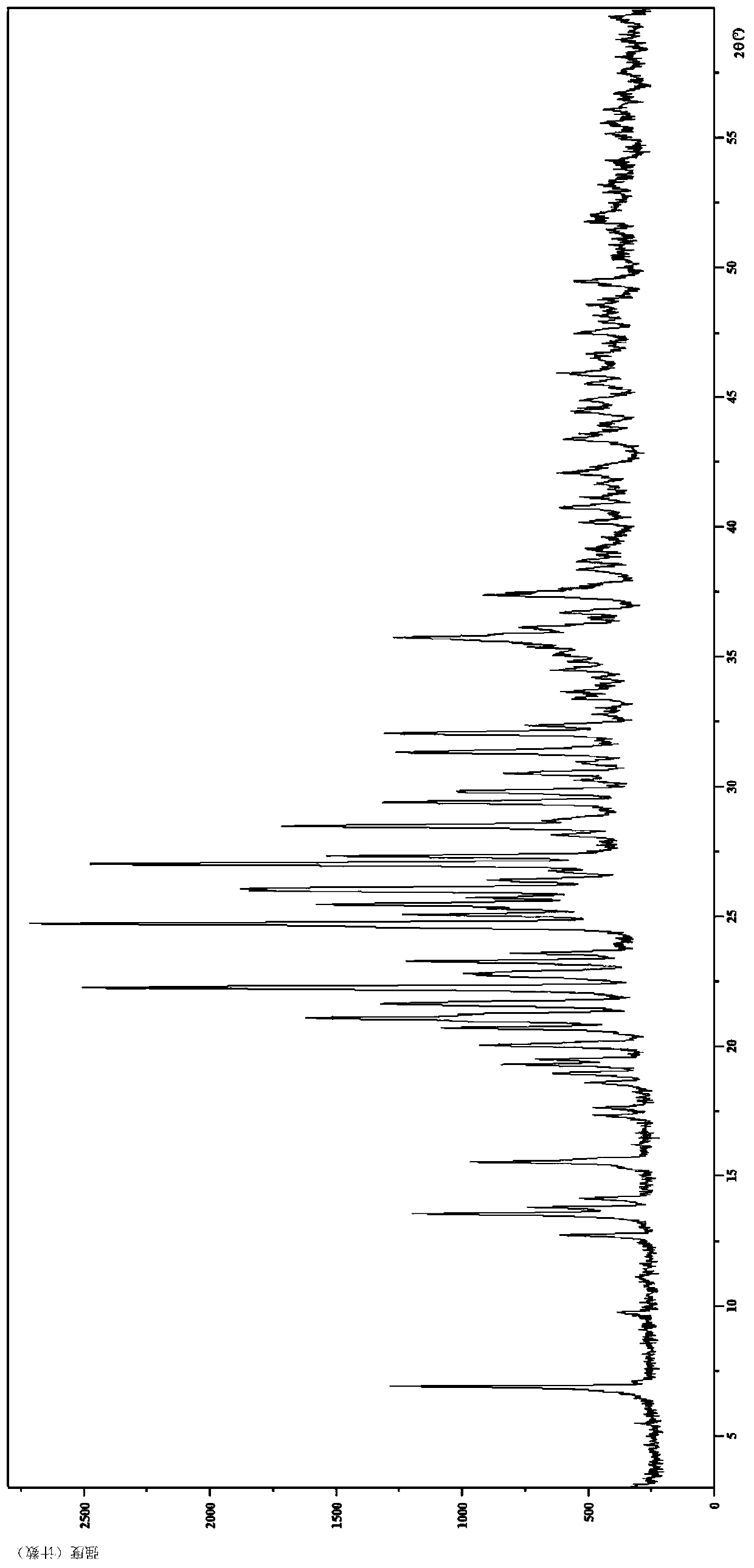
[0117] Embodiment 3 hydrobromide crystal form III
[0118] 1. Preparation of hydrobromide crystal form III
[0119] The compound 5-(4-cyclopropyl-1H-imidazol-1-yl)-N-(6-(6,7-dihydro-5H-pyrrolo[2,1-c][1,2,4 ]triazol-3-yl)pyridin-2-yl)-2-fluoro-4-methylbenzamide (51.1mg, 0.115mmol) was added to ethyl acetate (1.0mL), and beating at 65°C for 1 hour, Then add a self-made 1mol / L hydrobromic acid solution (0.25mL, 0.250mmol), keep warm for half an hour and then cool to room temperature naturally; filter with suction, and dry the filter cake under vacuum at 60°C overnight to obtain a white solid (46.5mg, 66.68%) .
[0120] 2. Identification of hydrobromide crystal form III
[0121] (1) Analysis and identification by Empyrean X-ray powder diffraction (XRPD): using Cu-Kα radiation, it has the following characteristic peaks expressed in angle 2θ: 6.86°, 9.70°, 11.06°, 12.68°, 13.51°, 13.75°, 14.10 °,15.53°,17.30°,17.61,18.56°,18.92°,19.27°,19.46°,20.02°,20.67°,21.04°,21.23°,21.6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com