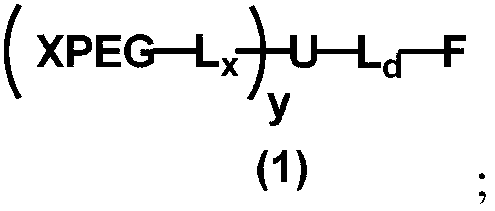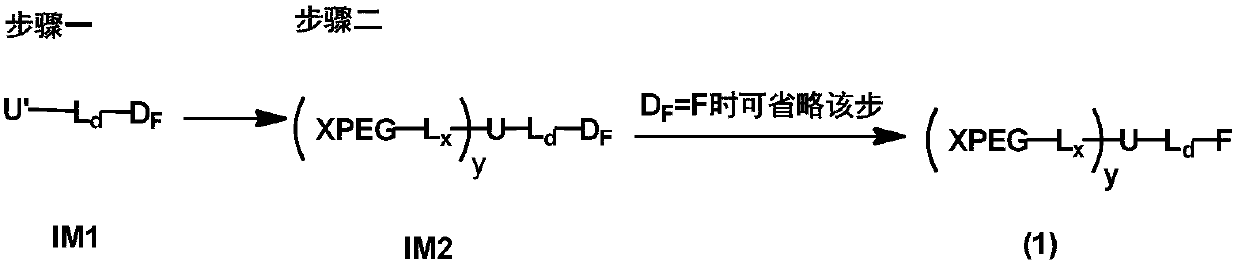Preparation method of monofunctionalized nonlinear polyethylene glycol
A polyethylene glycol and nonlinear technology, which is applied in the field of preparation of monofunctional nonlinear polyethylene glycol, can solve the problems of end group modification rate (substitution rate, limitation of functionalization rate, difficulty of separation and purification, large molecular weight, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0134] The present invention provides a kind of preparation method of monofunctional non-linear polyethylene glycol, the structural general formula (designed structure) of described monofunctional non-linear polyethylene glycol, as shown in formula (1):
[0135] Among them, F is a functional group, which contains a functional end group R 01 ; 01 with L d Directly connected, or via a divalent linker Z and L d connected;
[0136] L d is a covalent linking group containing a heteroatom; and L d Contains a covalent linker that can be formed via a coupling reaction, or L d and U form a covalent linking group that can be generated through a coupling reaction.
[0137] L d It can contain not only conventional divalent linking groups such as amide bonds, ester bonds, and urethane bonds, but also non-linear linking groups such as three-carbon bridges, such as the connections formed by the reaction of functional groups B5, B6, and E13 with disulfide bonds. Groups, such as reac...
Embodiment 1
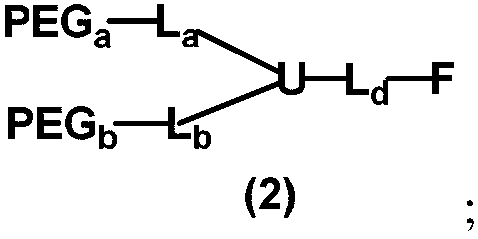
[0285] The monofunctional non-linear polyethylene glycol derivatives of embodiment 1 and embodiment 2 can also be classified as follows: XPEG has the general formula mPEG, two L xBoth are -CH 2 CH 2 OCONH-(the number of atomic intervals is 5), U is-(-CH 2 CH 2 CH 2 CH 2 ) CH-, L in Example 1 d Contains -CONH- and -NHCO-, F is -CH 2 CH 2 -MAL (wherein MAL is a maleimide group), the L of embodiment 2 d Contains -CONH-, F is -CH 2 CH 2 -CHO. The above two embodiments can also be attributed as follows: two L x Both are -CH 2 CH 2 OCONH-(atomic interval number is 5) and -CH 2 CH 2 OCONH(CH 2 ) 4 -(the number of atomic intervals is 9), and U is >CH-. It can also be assigned in the following way: XPEGs all have the general formula mPEG-CH 2 CH 2 -, L x Both are -OCONH- (the number of atomic intervals is 3), and U is -(-CH 2 CH 2 CH 2 CH 2 )CH-; or two L x are respectively -OCONH- (the number of atomic intervals is 3) and -OCONH (CH 2 ) 4 -(the number of at...
Embodiment 7
[0287] In Example 7, two L x Both are -CH 2 CH 2 COO-.
PUM
| Property | Measurement | Unit |
|---|---|---|
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com