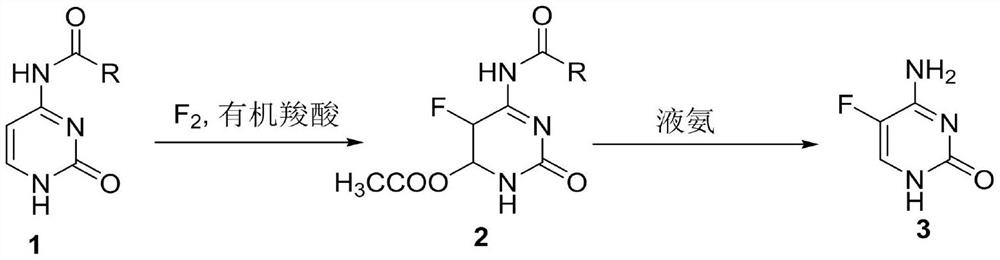
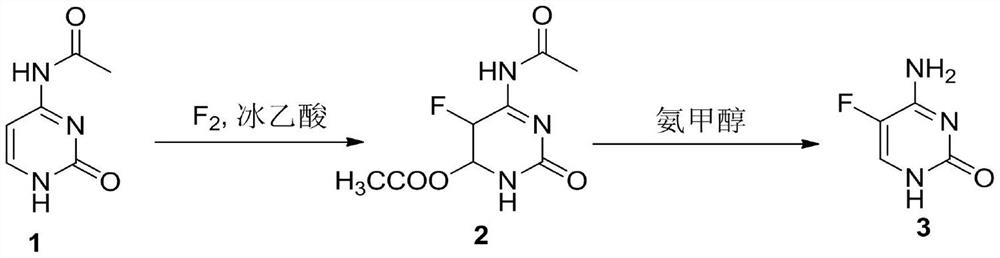
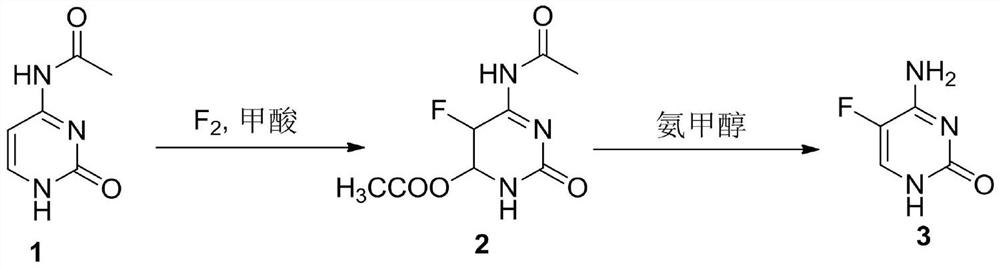
A method for synthesizing 5-fluorocytosine
A technology for flucytosine and acylcytosine, applied in the field of synthesizing 5-fluorocytosine, can solve the problems of high cost, high cost of hydrogen fluoride and formic acid, and achieve the effects of safe production process, shortened steps, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025]
[0026] The first step, N 4 -40g (0.26mol) of acetylcytosine was put into the reaction flask, 400mL of glacial acetic acid was added and then heated to 40°C, 9.9g (0.26mol) of 20% fluorine gas (80% nitrogen gas) was slowly introduced, and the liquid phase tracked the reaction to the raw material Basically disappeared, cooled and concentrated to recover glacial acetic acid, added 200 mL of methanol to the remaining oil, stirred at 20° C. for 30 min, a large amount of solid was precipitated, suction filtered to obtain the intermediate, and dried to obtain 54 g (0.23 mol) of intermediate 2.
[0027] In the second step, in the reaction kettle, 54g of intermediate 2 was added, then 200mL of ammonia methanol was added and the temperature was raised to 50°C for a closed reaction for 5h to cool down, the ammonia methanol was concentrated and recovered, and the residue was purified by adding 400mL of water to obtain 26.7g of 5-fluorocytosine ( 0.21 mol), HPLC: 99.9%. 1 HNMR...
Embodiment 2
[0029]
[0030] The first step, N 4 -40 g (0.26 mol) of acetylcytosine was put into the reaction flask, 400 mL of formic acid was added and then the temperature was raised to 40° C., 9.9 g (0.26 mol) of 20% fluorine gas (80% nitrogen gas) was slowly introduced, and the liquid phase tracked the reaction until the raw materials were basically Disappeared, cooled and concentrated to recover formic acid, added 200 mL of methanol to the remaining oil, stirred at 20° C. for 30 min, a large amount of solid was precipitated, suction filtered to obtain the intermediate, and dried to obtain 52 g (0.22 mol) of intermediate 2.
[0031] In the second step, 52g of intermediate 2 was added in the reaction kettle, then 200mL of 20% ammonia methanol was added, and the temperature was raised to 50°C for a closed reaction for 5h to cool down, the ammonia methanol was concentrated and recovered, and the residue was purified by adding 400mL of water to obtain 26g of 5-fluorocytosine (0.22 mol),...
Embodiment 3
[0033]
[0034] In the first step, 40 g (0.26 mol) of N4-acetylcytosine was put into the reaction flask, 400 mL of glacial acetic acid was added, and then the temperature was raised to 40° C. The phase tracking reaction was carried out until the raw materials basically disappeared. The glacial acetic acid was recovered by cooling and concentration. 200 mL of methanol was added to the remaining oil, and a large amount of solid was precipitated after stirring at 20° C. for 30 min.
[0035] In the second step, 54g of intermediate 2 was added in the reaction kettle, then 200 mL of 20% ammonia methanol was added, and the temperature was raised to 50 °C for a closed reaction for 5 h to cool down, the ammonia methanol was concentrated and recovered, and the residue was purified by adding 400 mL of water to obtain the product 5-fluorocytosine 26.9 g (0.21 mol), HPLC: 99.6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com