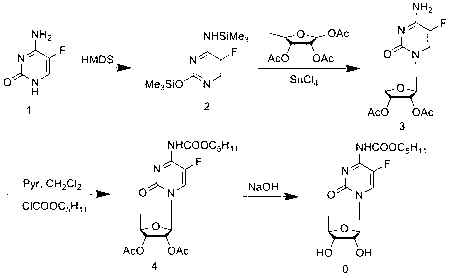
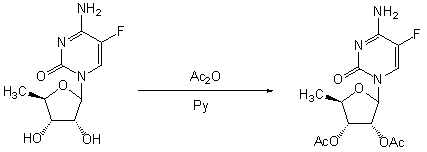
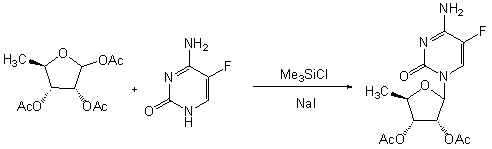
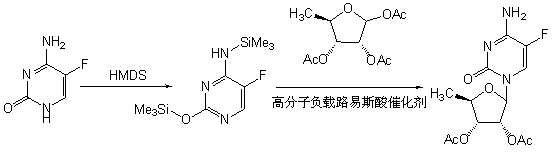
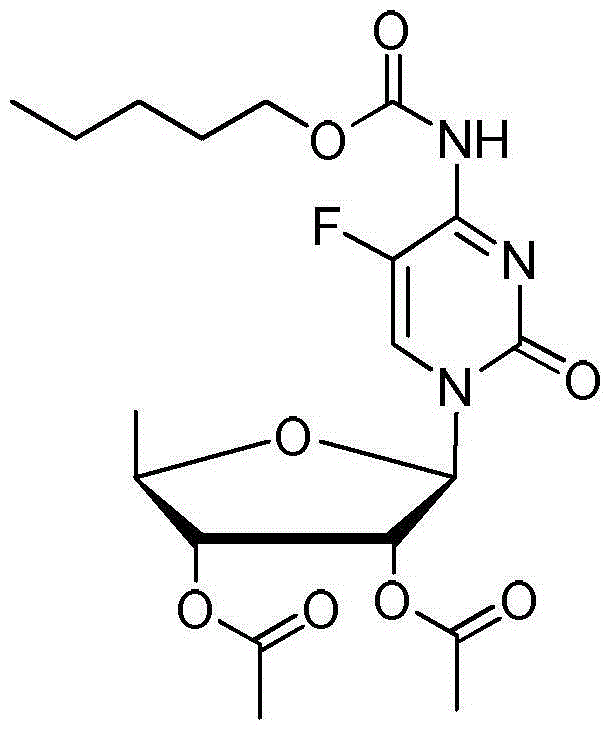
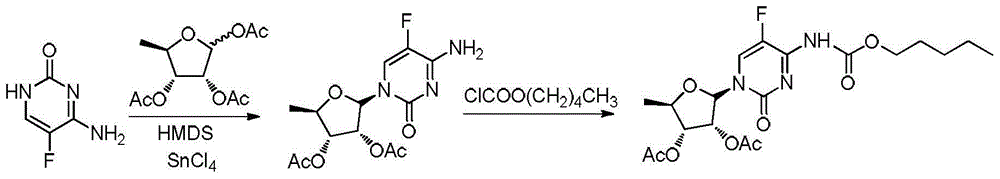
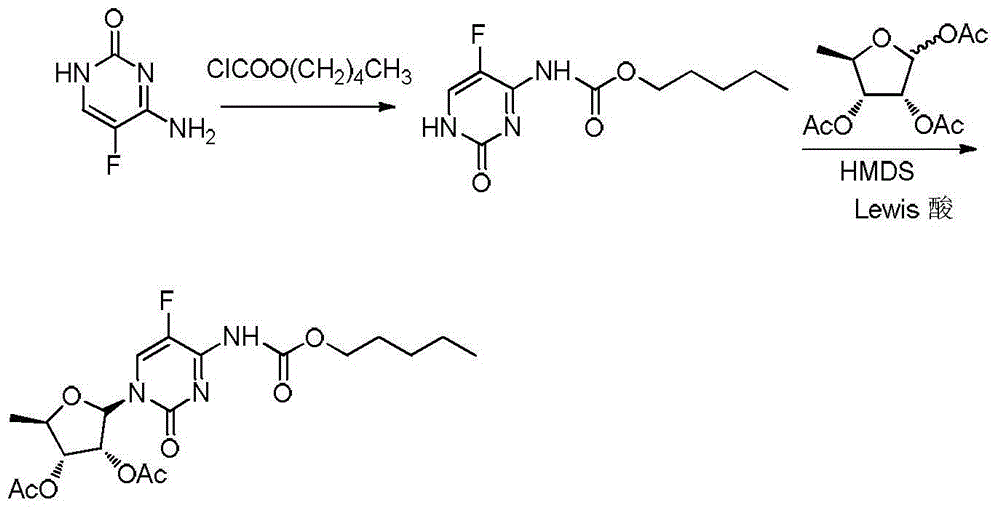
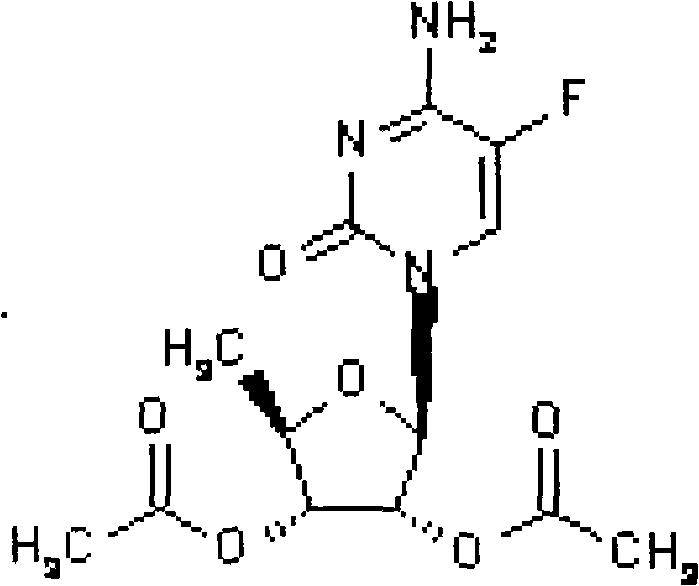
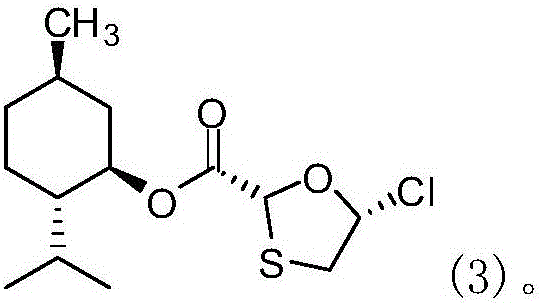
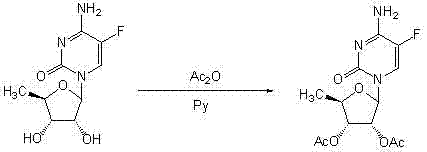
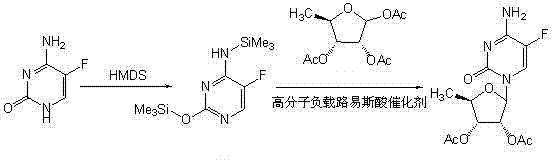
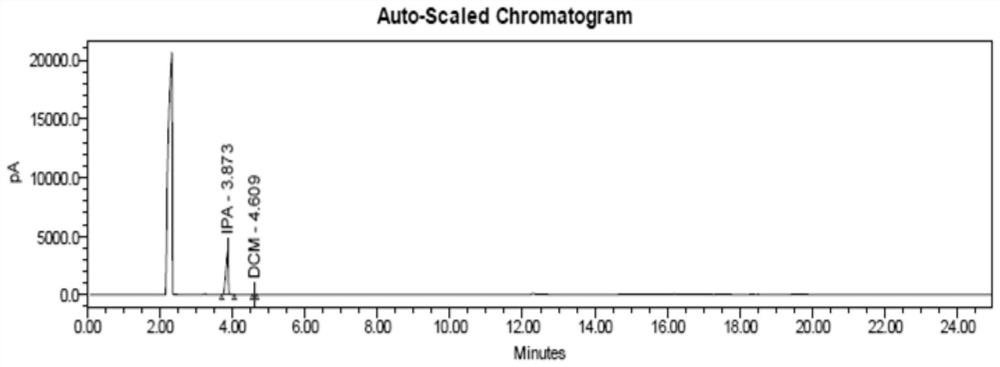
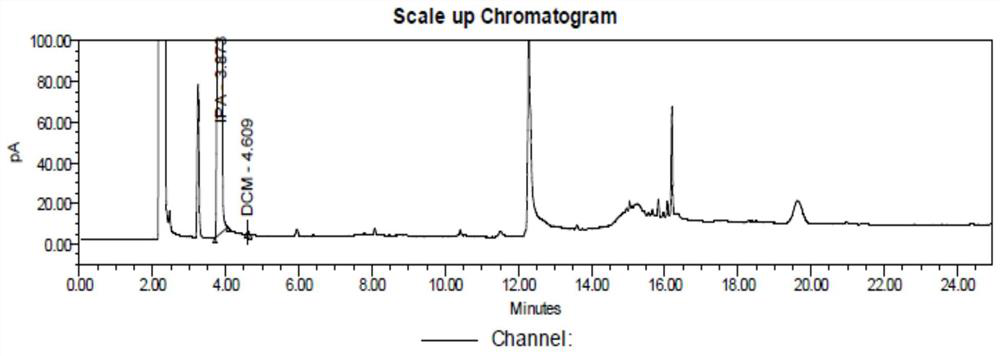
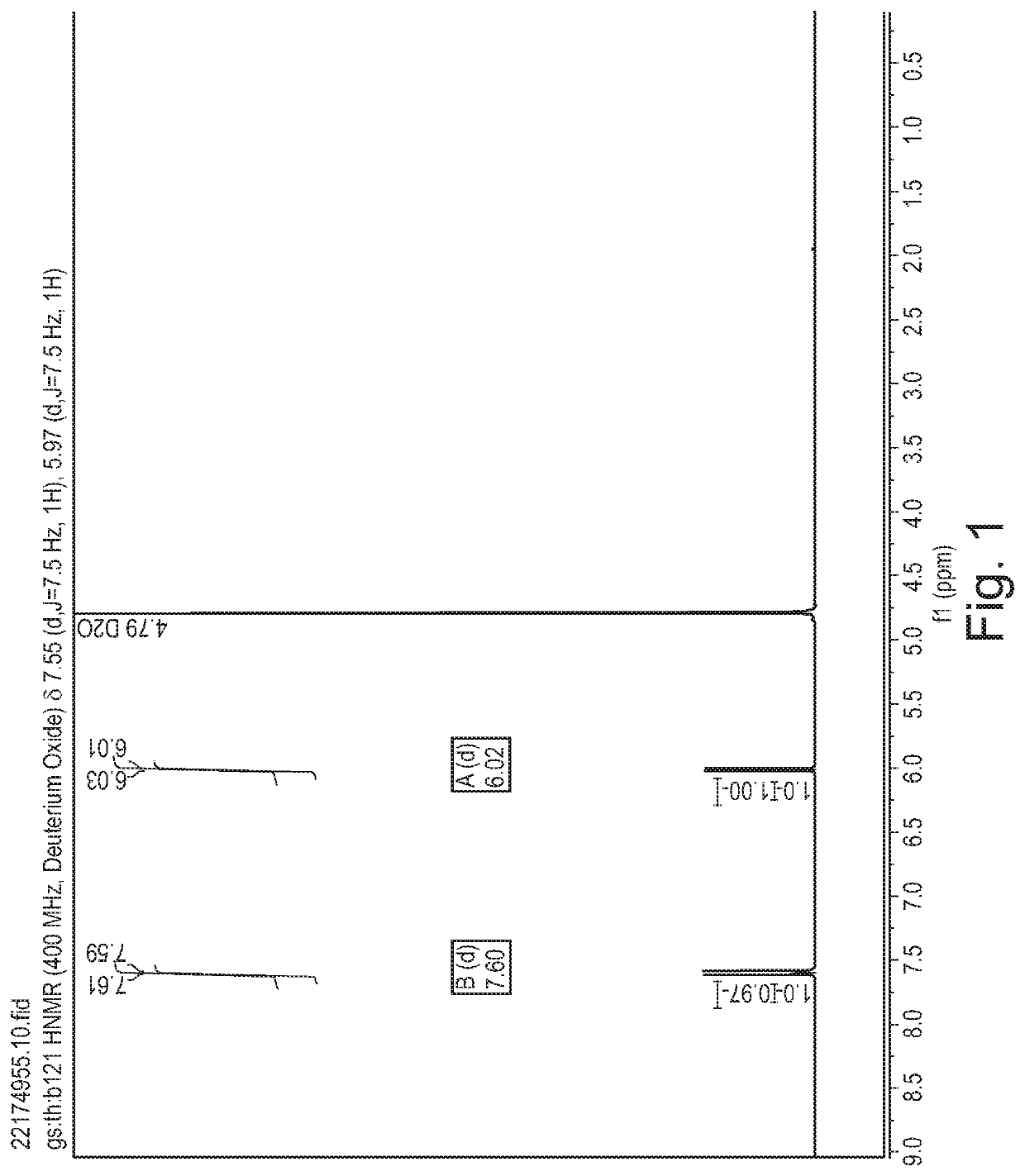
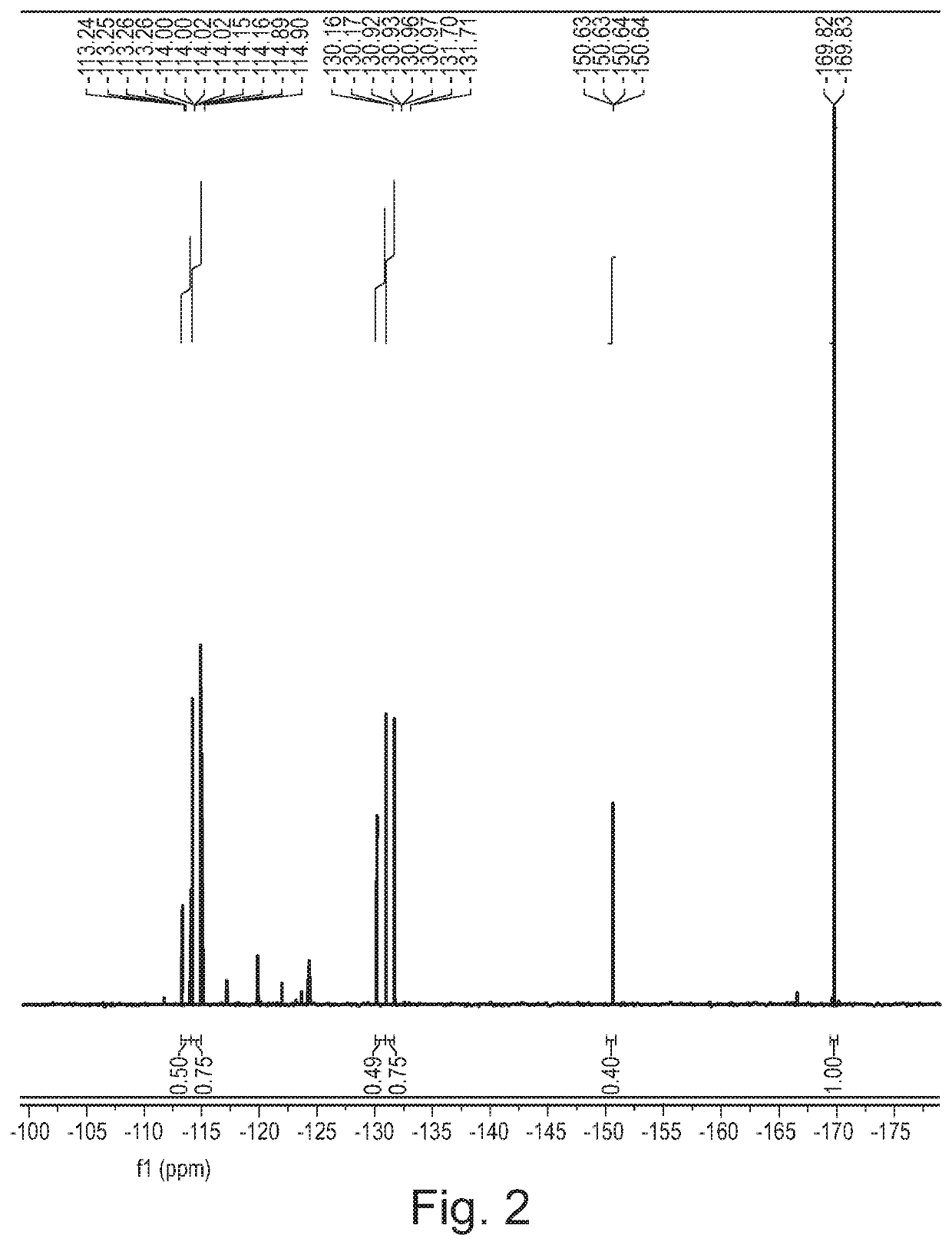
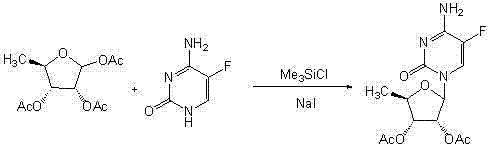Patents
Literature
39 results about "Flucytosin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
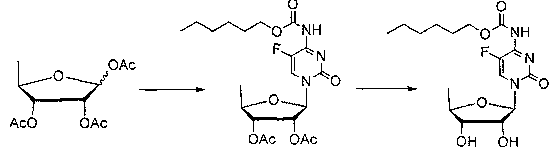
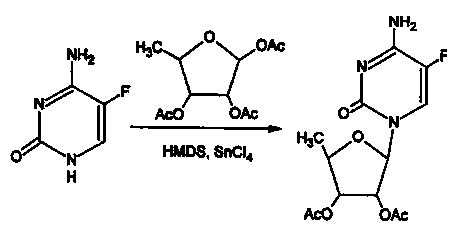
Method for preparing capecitabine and hydroxyl derivative intermediate thereof
InactiveCN102241721AMild reaction conditionsSimple processSugar derivativesSugar derivatives preparationChemical reactionControllability
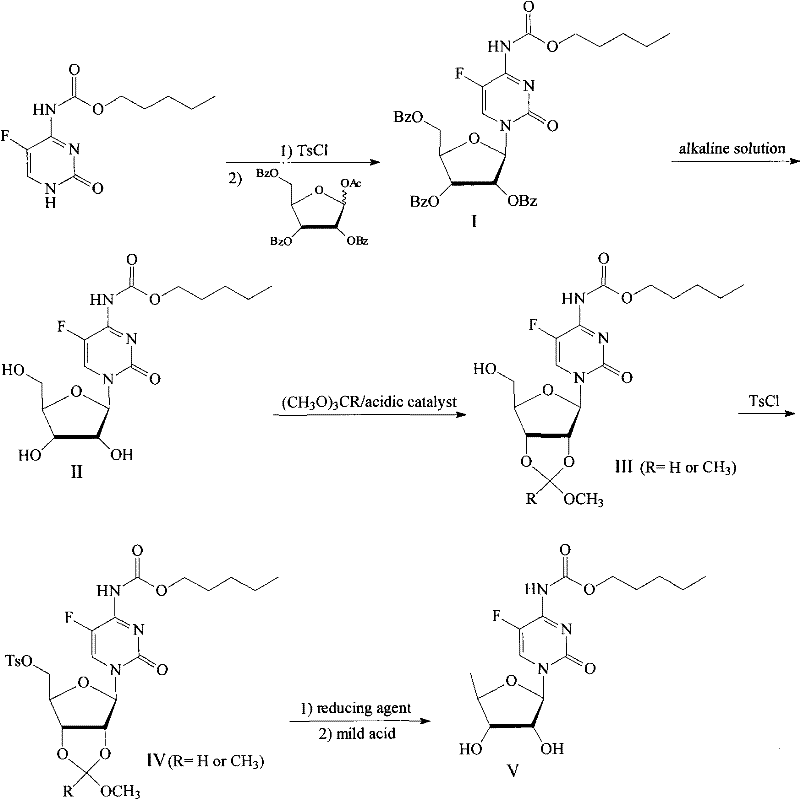
The invention discloses a method for preparing an anticancer medicine capecitabine and a hydroxyl derivative key intermediate thereof, and belongs to the technical field of pharmaceutical chemistry. The method is characterized in that: N-[(p-pentyloxy)carbonyl]-5-flurocytosin is taken as an initial raw material, and is subjected to five steps of chemical reactions to form the capecitabine. The preparation method has the advantages of reasonable sequence, readily available raw materials, mild reaction conditions, high process controllability, high yield and low cost. The crude intermediate has high purity, complicated purification treatment is avoided, the capecitabine obtained in a later stage can reach the standard of United States Pharmacopeia, and the method is more suitable for industrial production.
Owner:JIANGNAN UNIV
Novel technology for synthesis of capecitabine
InactiveCN103288905ASuitable for industrialized mass productionHigh yieldSugar derivativesSugar derivatives preparationSodium methoxideMeth-
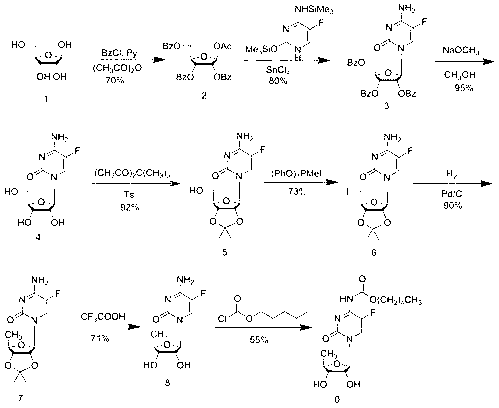
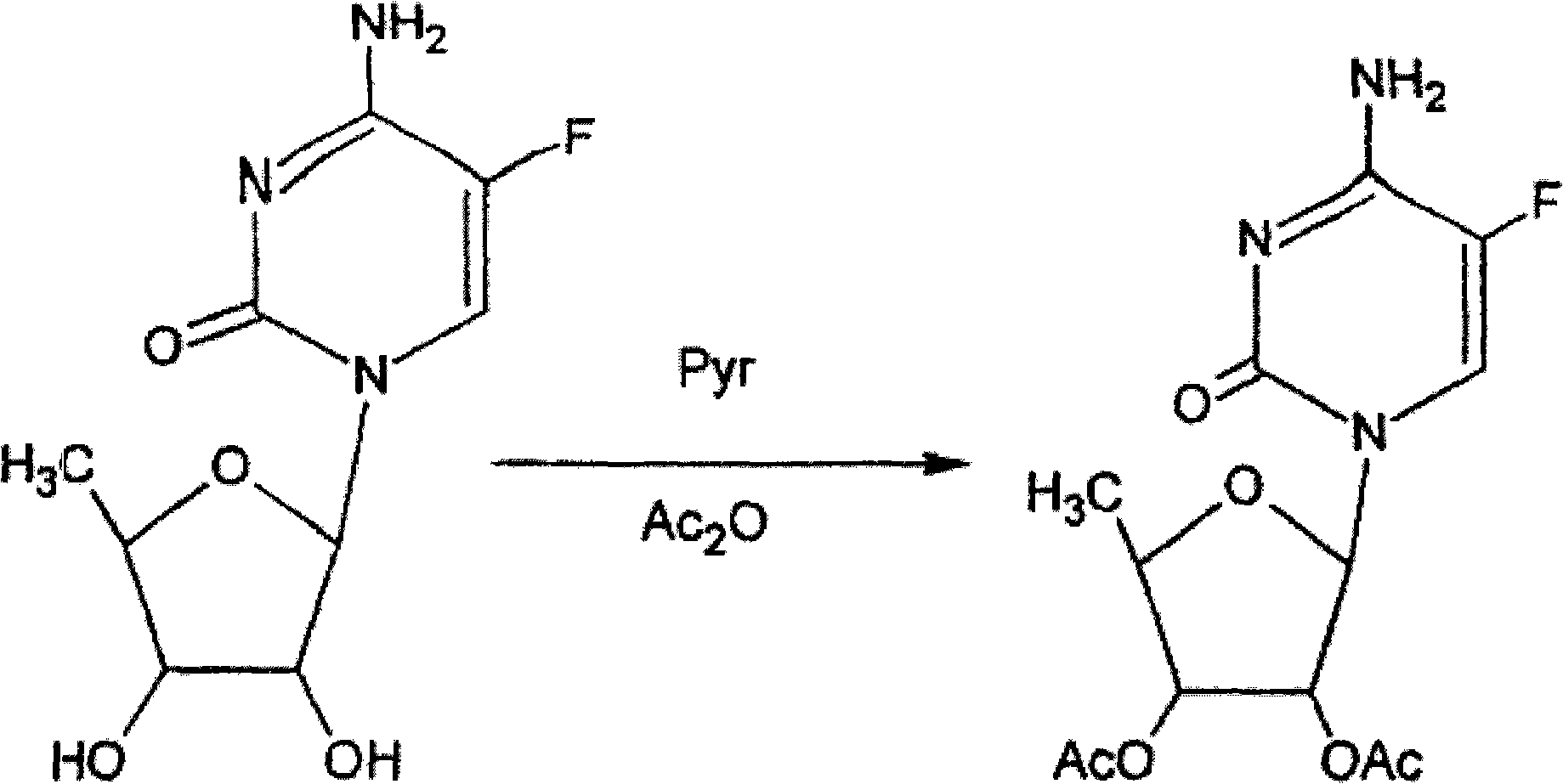
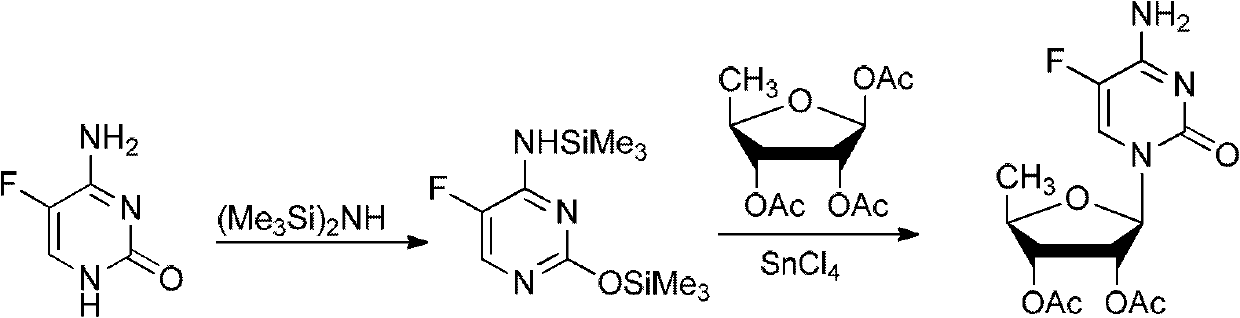
The invention relates to a novel technology for synthesis of capecitabine. The technology is characterized in that: 5-fluorocytosine protected by trimethyl silicon is taken as raw material; and the capecitabine is obtained after condensation, esterification and deacetylation. The Reaction sequence is more economically reasonable, the synthetic route is short, the cost is low, the operation is simplified, the yield is high, the synthetic period is short, the quality of intermediates can be controlled, solvents used in reaction are few, pollution to the environment is little, and the technology is suitable for industrial production. Comparing the technology with the prior art for capecitabine production, trimethylsilyl trifluoromethanesulfonate (TMSOTf) which replaces a heavy metal agent stannic chloride is used as a condensing agent for glycosylation (condensation), and a sodium methoxide / methanol system replaces an ammonia gas / methanol system for deacetylation, so that the production yield is increased, and heavy metal residues of the products and the environmental pollution are reduced. The overall yield of the technology of the invention reaches 59%, the purity of the production is high and meets the standards of the United States Pharmacopeia.
Owner:北京博时安泰科技发展有限公司
Preparation method of 2',3'-bi-O-acetyl-5'-deoxy-5-fulurocytidine
ActiveCN102993253ALess impuritiesShort reaction timeSugar derivativesSugar derivatives preparationPtru catalystCombinatorial chemistry
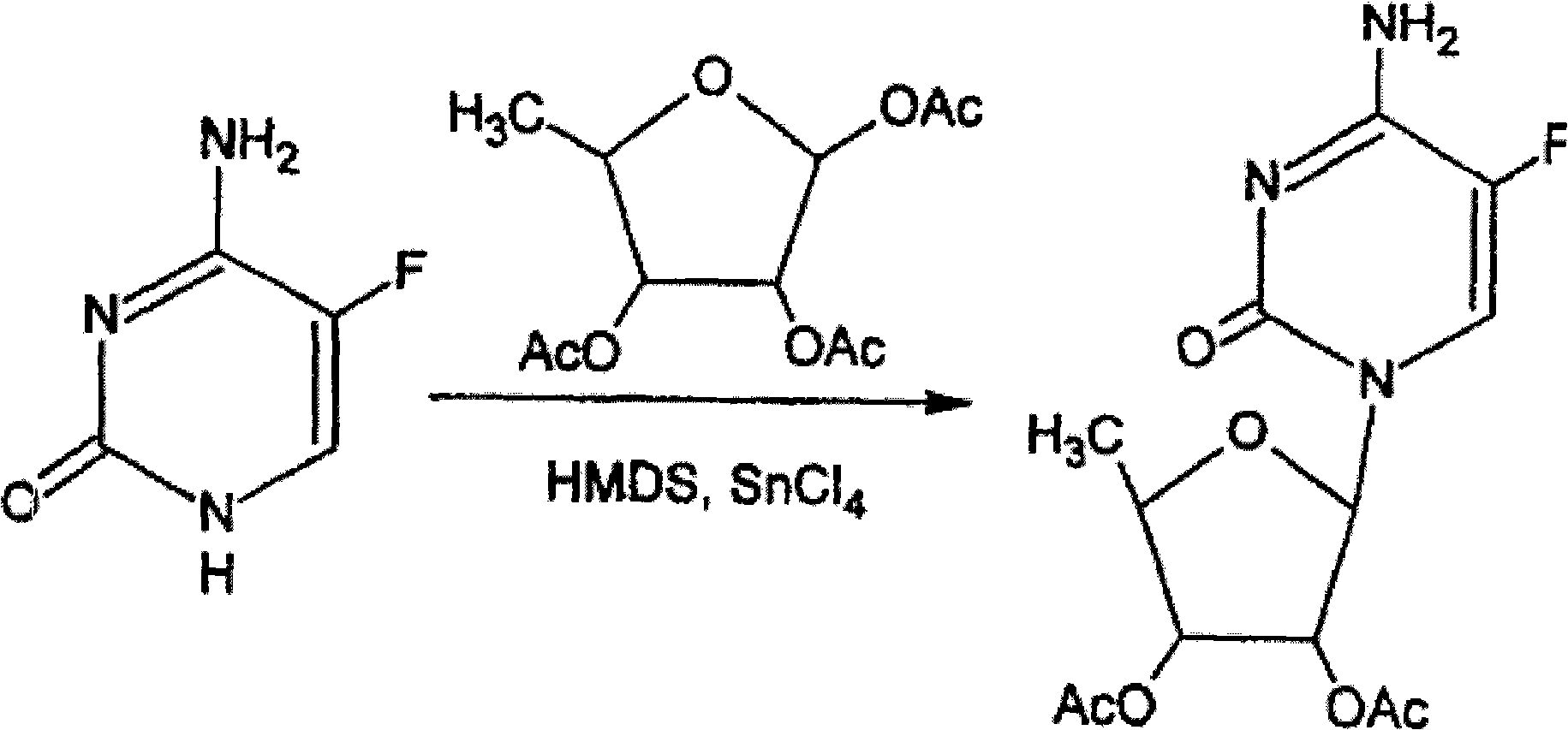
The invention discloses a preparation method of 2',3'-bi-O-acetyl-5'-deoxy-5-fulurocytidine. The preparation method comprises the following steps of: carrying out glycosylation reaction on 1,2,3-tri-O-acetyl-5-deoxyribose and bi-silanized 5-fluorocytosine under the catalysis of polymer-supported lewis acid which can be separated and reused for multiple times to obtain reaction liquid, and separating and purifying the reaction liquid to obtain a high-purity important capecitabine intermediate, namely the 2',3'-bi-O-acetyl-5'-deoxy-5-fulurocytidine. According to the preparation method, the catalyst is stable and efficient, can be easily separated from a reaction system and is free of pollution and single in stereoselectivity; the content of an Alpha isomer in the reaction liquid is smaller than 1.4 percent and can be removed by recrystallization; and the 2',3'-bi-O-acetyl-5'-deoxy-5-fulurocytidine has the optical purity up to 99.6 percent and is suitable for industrial production.
Owner:ZHEJIANG XIANFENG TECH
Synthetic method for capecitabine key intermediate
ActiveCN104926901AHigh yieldControl ratioSugar derivativesSugar derivatives preparationChloroformateChloroform
The invention discloses a synthetic method for capecitabine key intermediate 2`,3`-O-diacetylpyridine-5`-deoxygenation-5-fluorine-N4-[(pentyloxy) carbonyl] cytidine. The synthetic method for the capecitabine key intermediate comprises the following steps that 1, 5- fluorocytosine, an acid-binding agent, chloroform, water and phase transfer catalyst are mixed, pentyl chloroformate is added dropwise under stirring, and the chloroform solution of (5-fluorine-2-oxo-1,2-dihydropyrimidine-4-base) amylcarbamate is obtained; 2, 1,2,3-three-O-acetyl-5-deoxygenation-6- ribofuranose is added into the chloroform solution obtained in the step 1, lewis acid is added dropwise, the reaction is performed for 2-10 hours after adding, and the capecitabine key intermediate is obtained after post-processing. The synthetic method is simple and convenient in operation, a silicane protective agent and intermediate product purification are not needed, the high yield of finished products is achieved, the proportion of alpha isomer in the products is effectively controlled, and compared with literature data, the purity of the obtained products is greatly improved.
Owner:广安凯特制药有限公司
Preparation method of 2',3'-di-O-acetyl-5'-deoxy-5-fluorocytidine
InactiveCN102250175ASugar derivativesSugar derivatives preparationTrimethylsilyl trifluoromethanesulfonate5-fluorocytidine
The invention provides a preparation method of capecitabine intermediate 2',3'-di-O-acetyl-5'-deoxy-5-fluorocytidine, which is suitable for actual industrial big production and has the advantages of high yield and quality and fine stability. The preparation method of capecitabine intermediate comprises the step of innovatively using a trifluoromethanesulfonic acid trimethylsilyl ester catalyst as the silanization agent of 5-fluorocytosine. The method has the advantages of high yield and good quality, and the process is simple and easy to operate.
Owner:NANJING VARSAL MEDICINE TECH DEV
Bacterial with high-yield of nucleoside phosphorylase and method for synthesizing arabinose nucleoside
InactiveCN101113420AIncrease vitalityImprove conversion rateBacteriaFermentationFlucytosineNucleoside phosphorylase
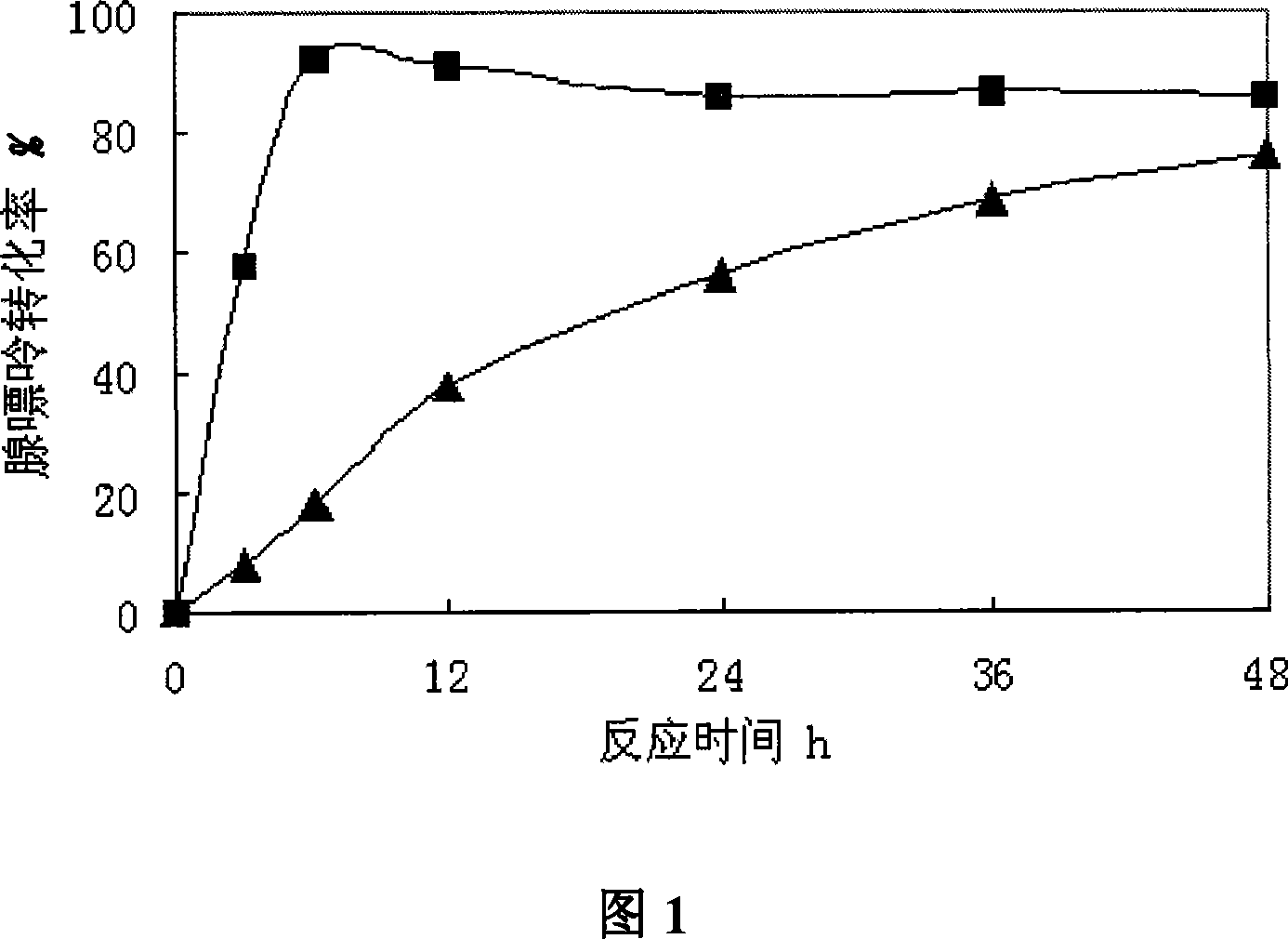
A compound method of high-yield nucleoside phosphorylase strains and arabinose nucleoside pertains to biochemical engineering field, in particular to the high-yield nucleoside phosphorylase strains and a method for compounding arabinose purine nucleoside with the strains by an enzyme method. The invention aims at solving a technical problem for providing the strains of the high-yield nucleoside phosphorylase and strains of uridine phosphorylase and the method for producing the arabinose purine nucleoside with the strains. The invention discloses enterobacter aerogenes with a preservation number of CGMCC No.2035 and the method for producing the arabinose purine nucleoside with the strains, and the invention comprises steps that (1) the enterobacter aerogenes DWOQ-58 of the invention is cultured and collected, and (2) the enterobacter aerogenes DWOQ-58 is contacted with arabinose donor and receptors of purine base. The strains of the invention are rich in vigor and resists 5-flucytosine with an average conversion rate of more than 80 percent in general and the reaction time of the invention is shortened to less than 12 hours.
Owner:SHANGHAI WEIPING BIOLOGICAL TECH
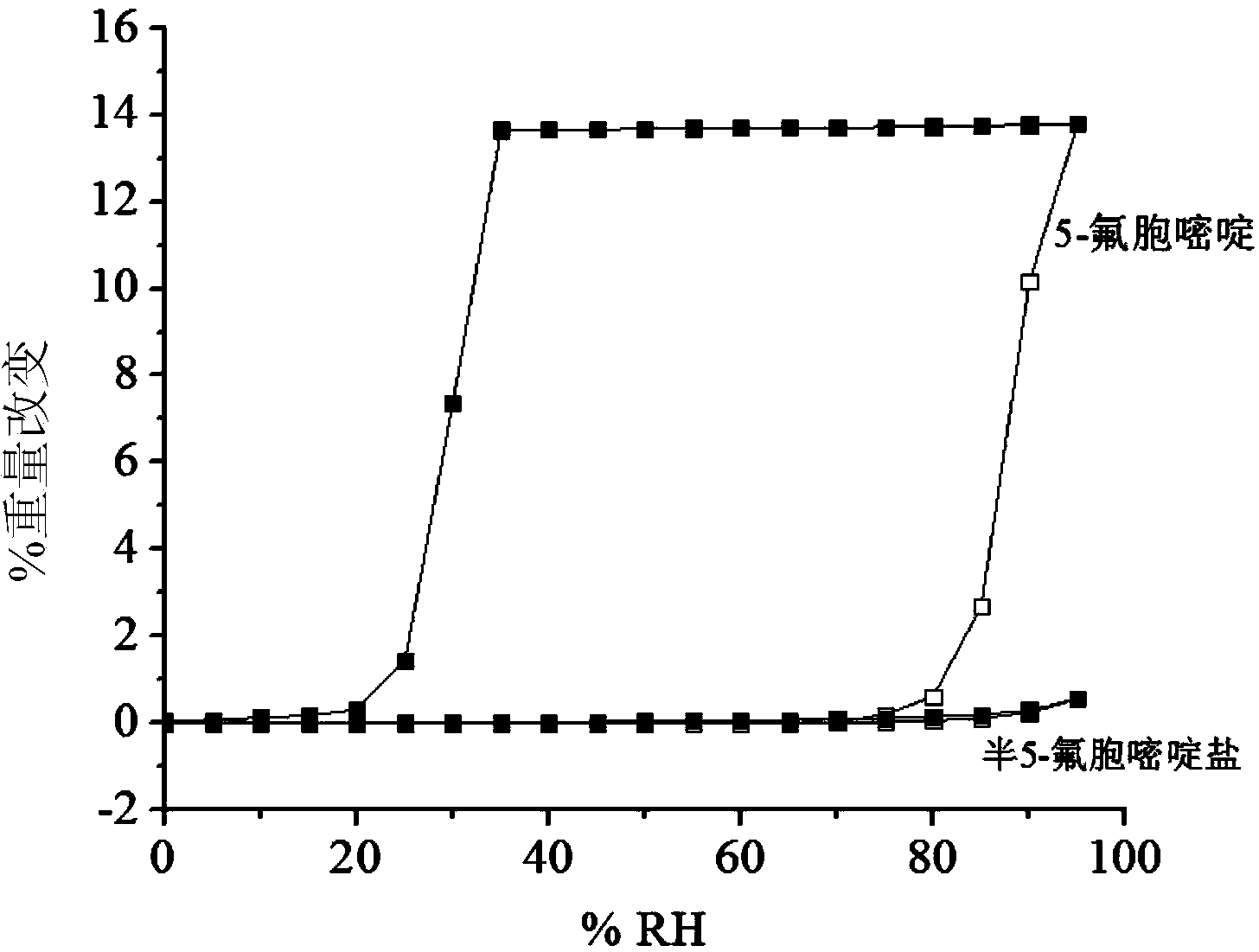
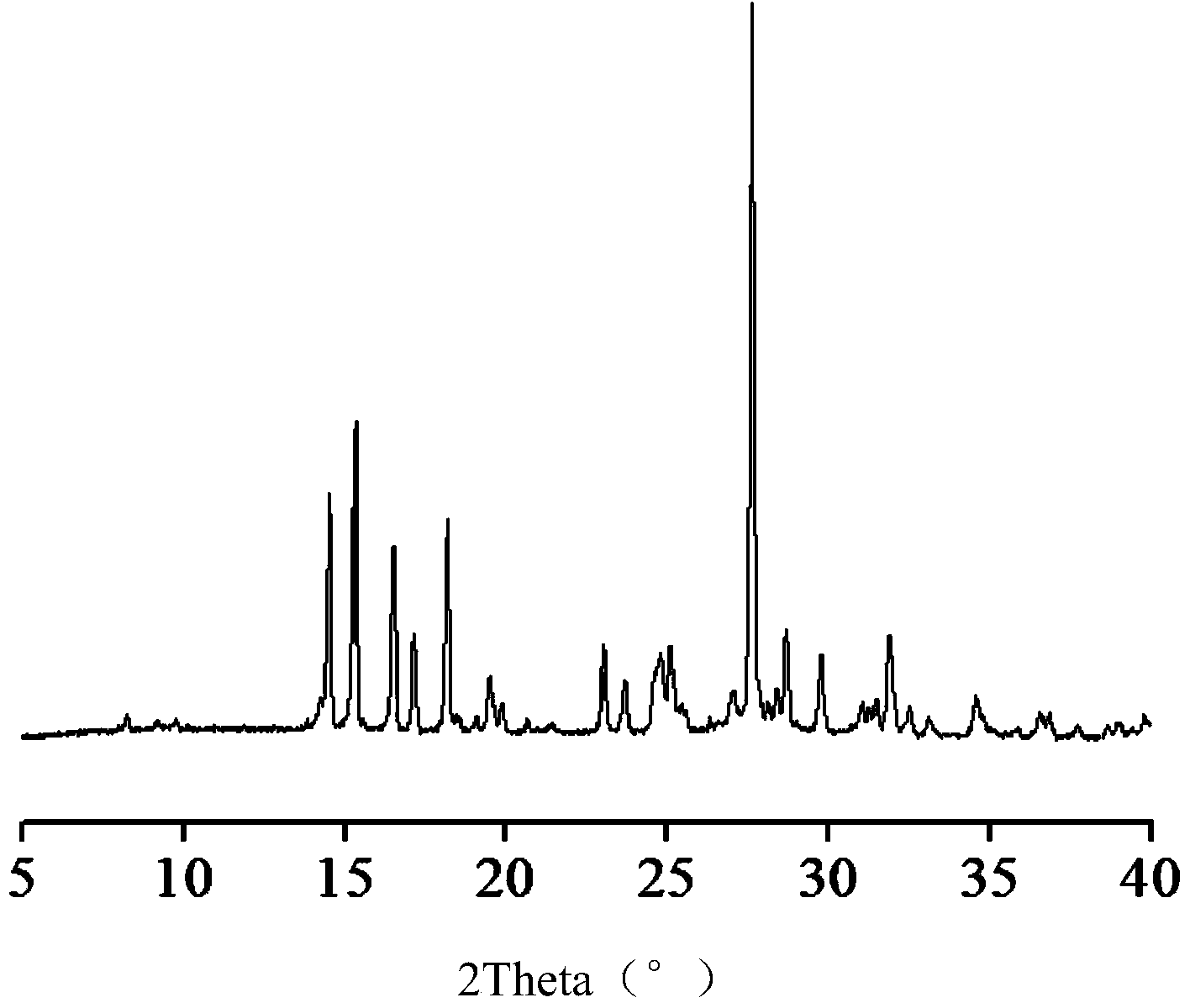
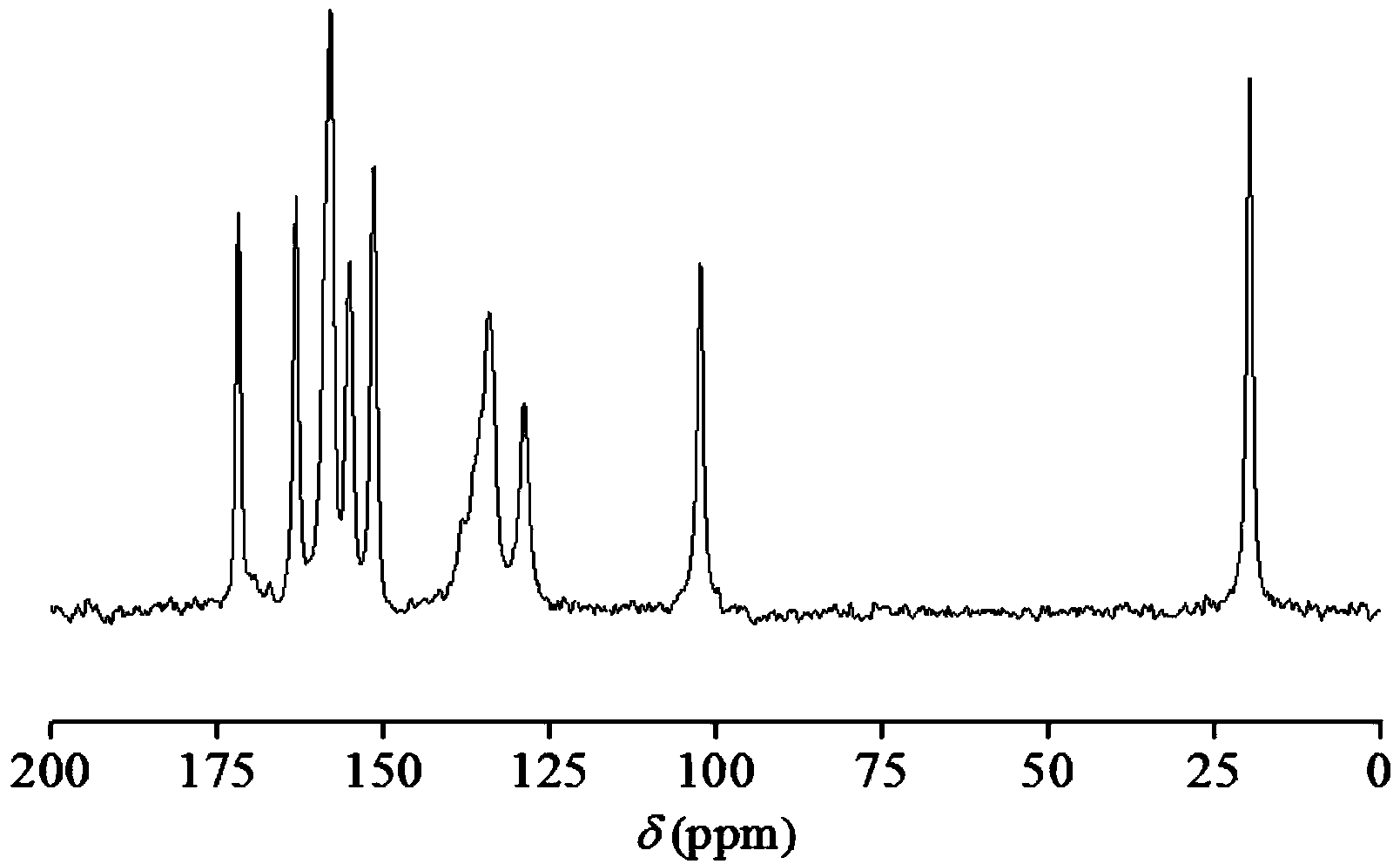
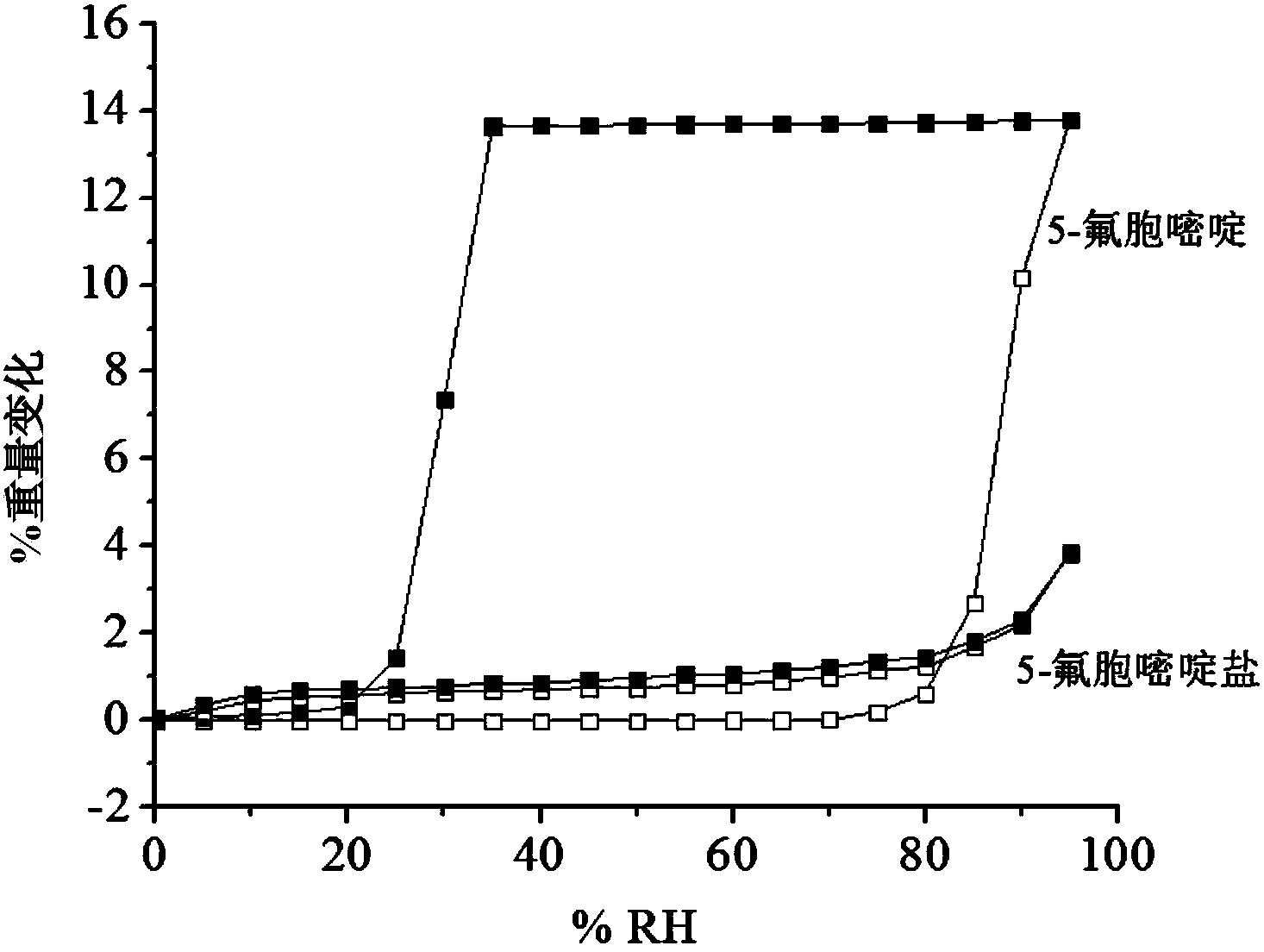
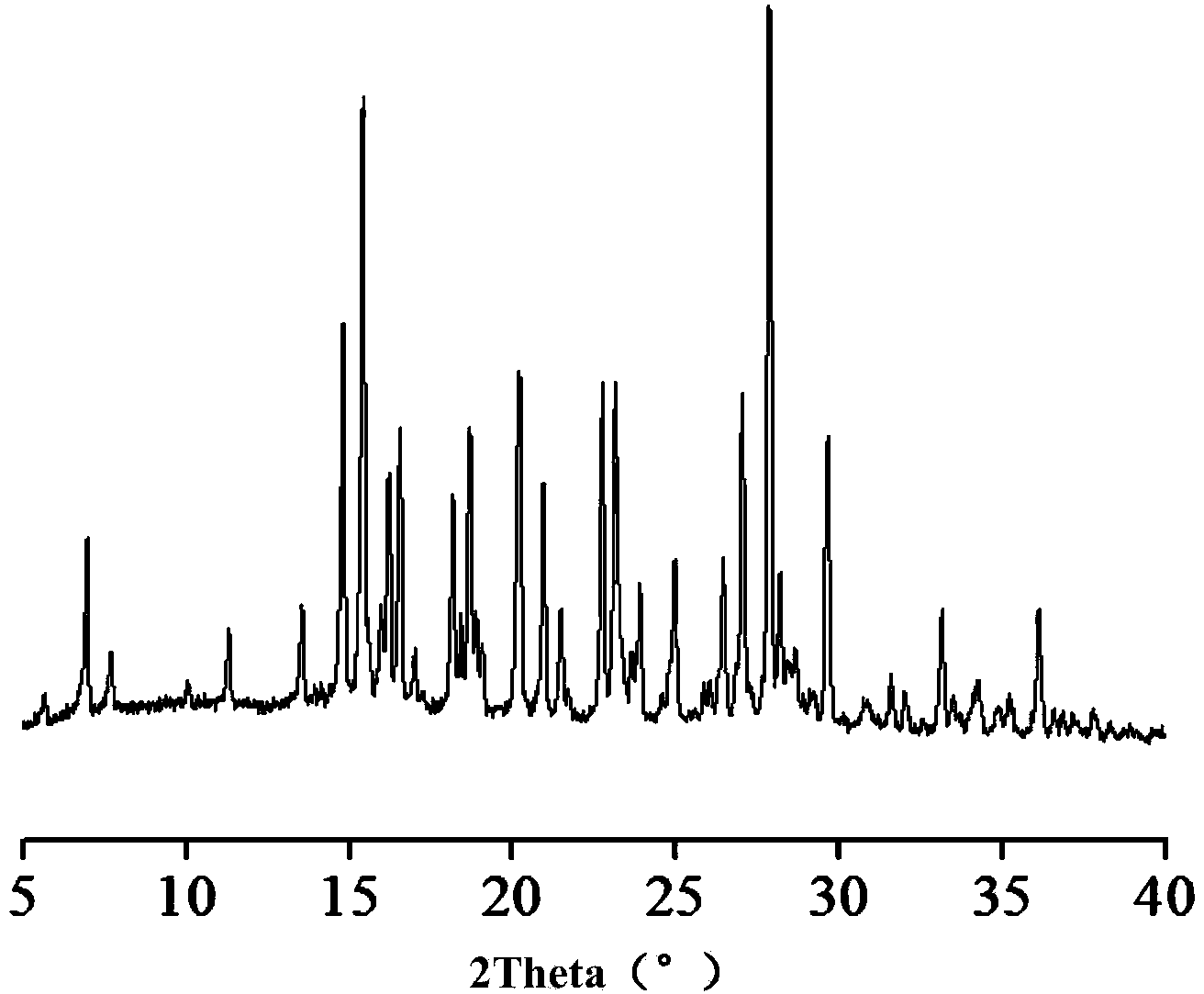
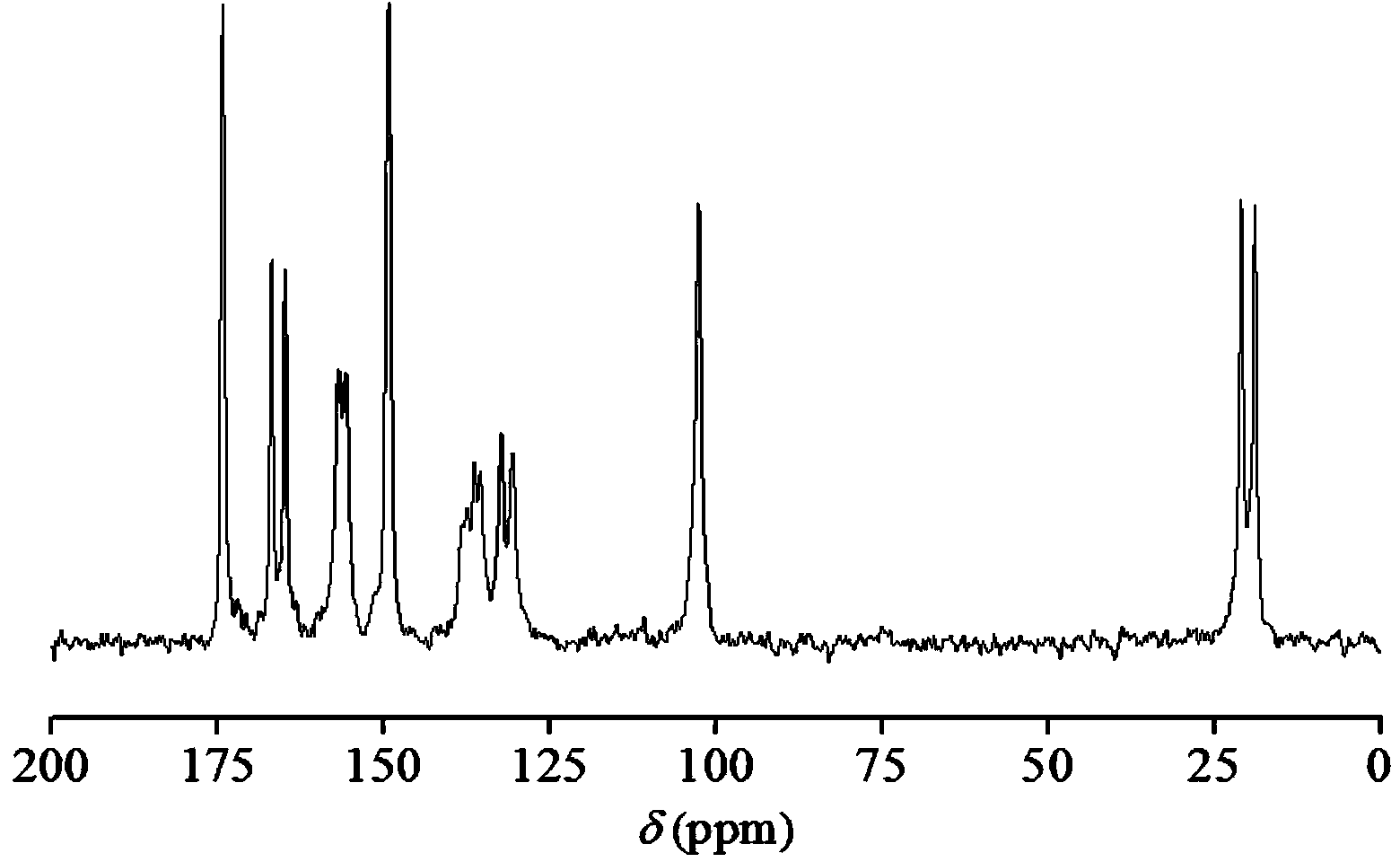
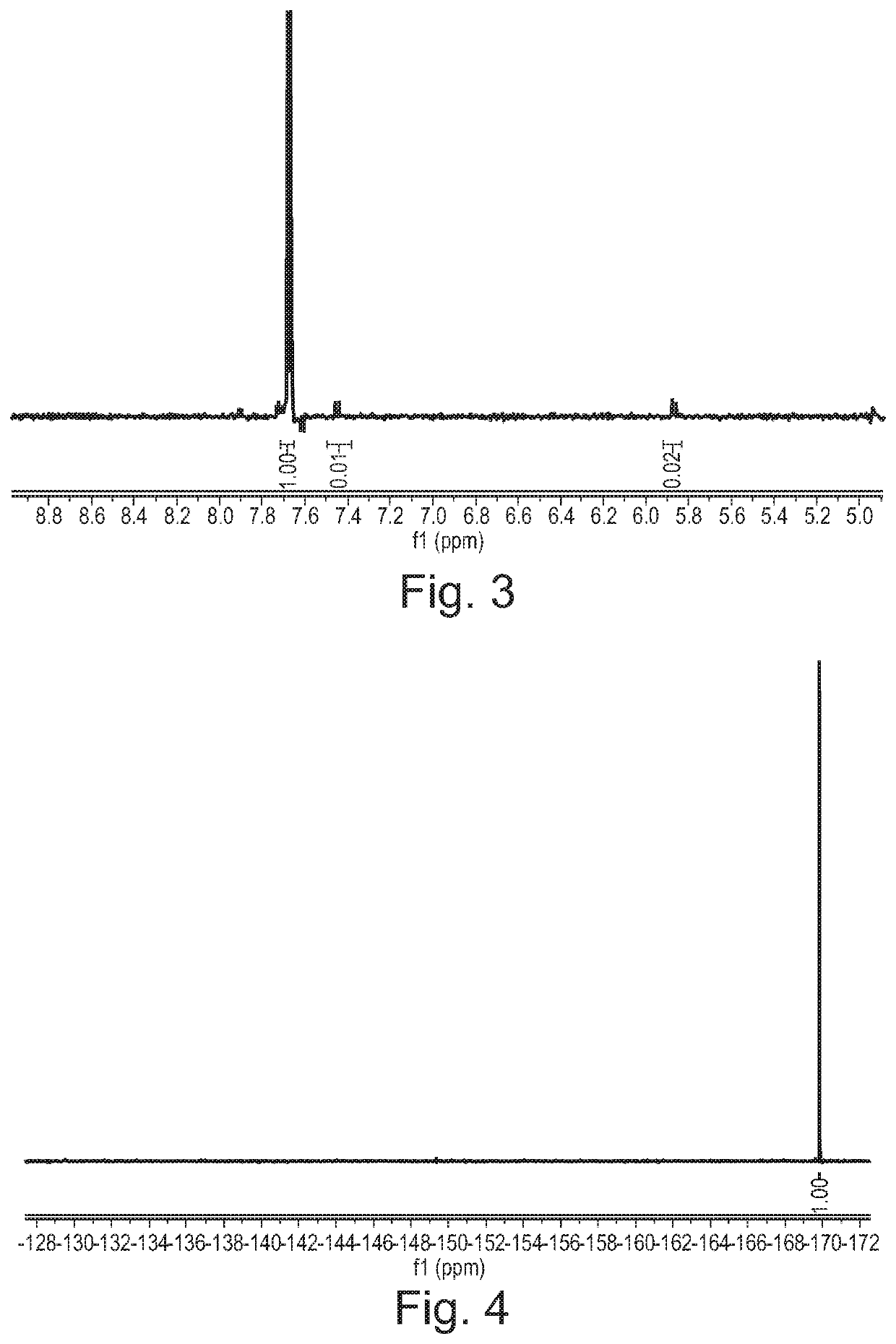
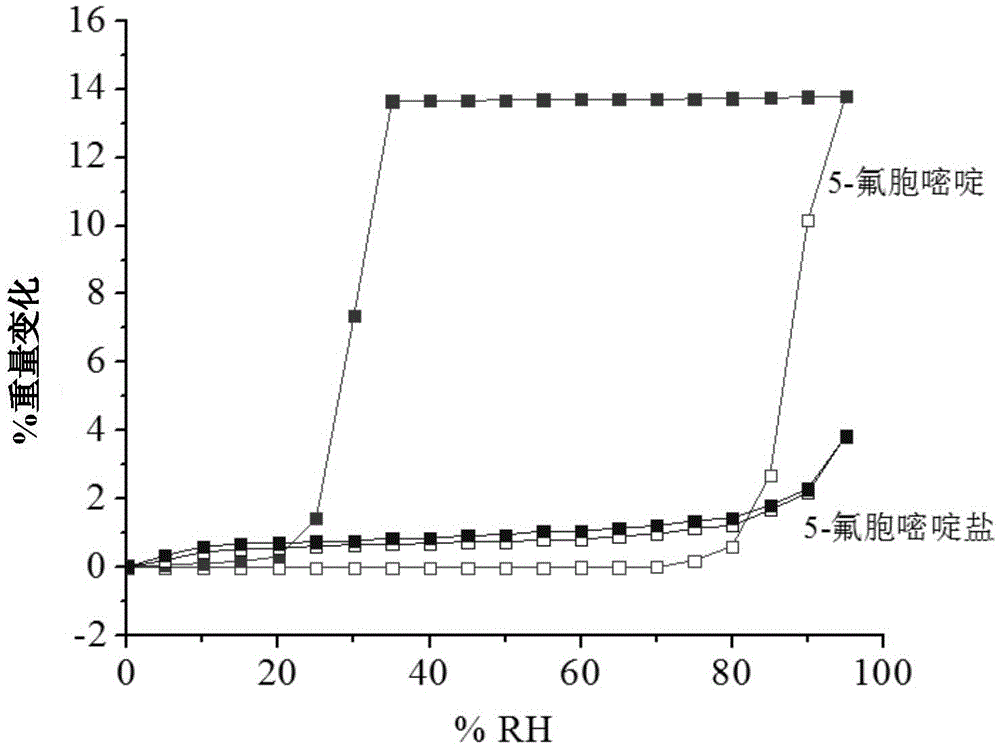
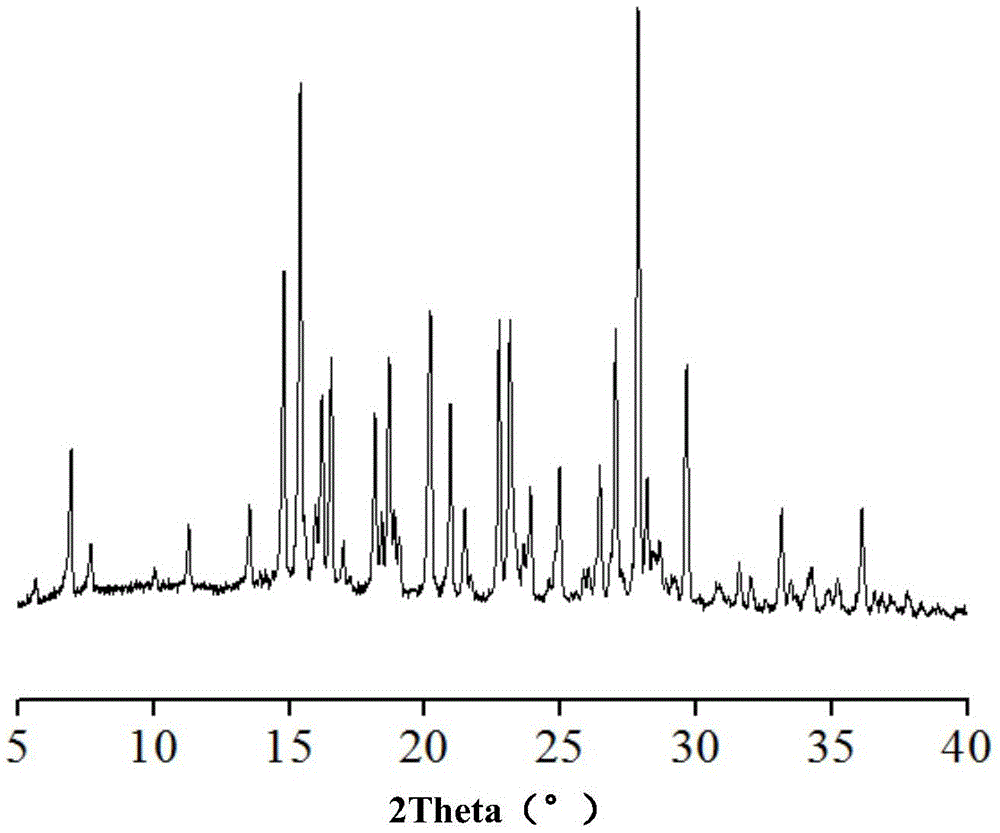
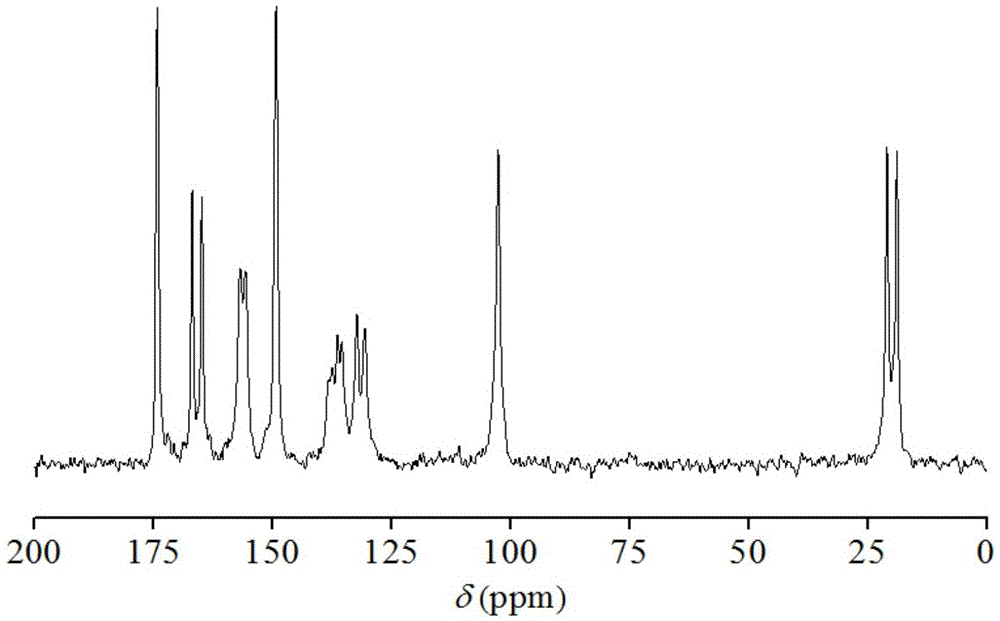
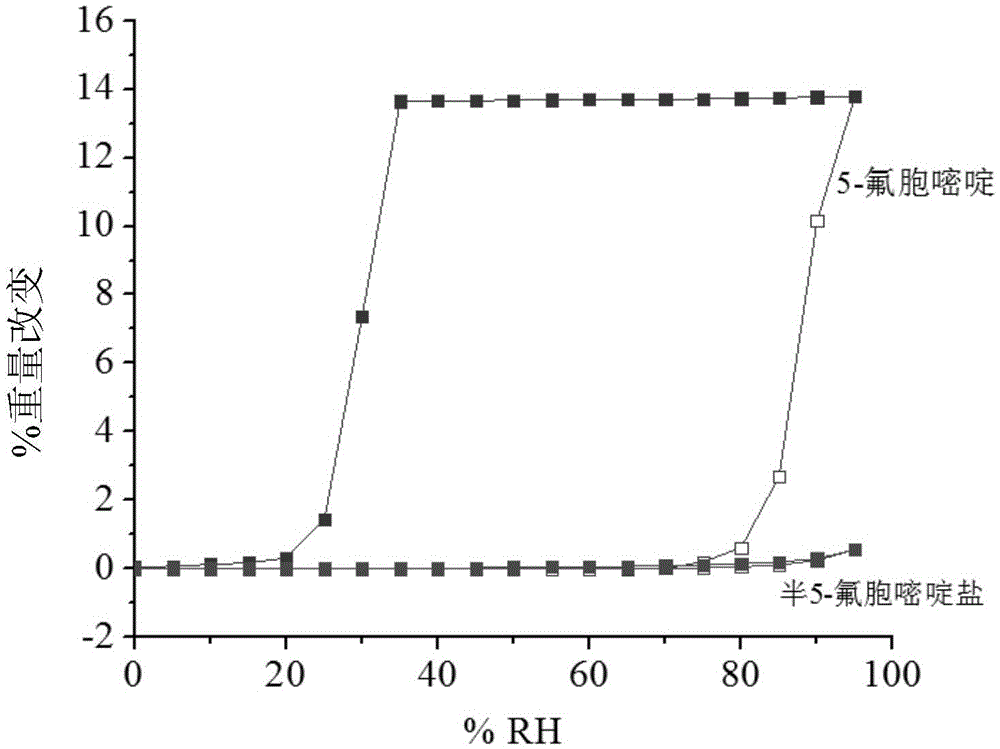
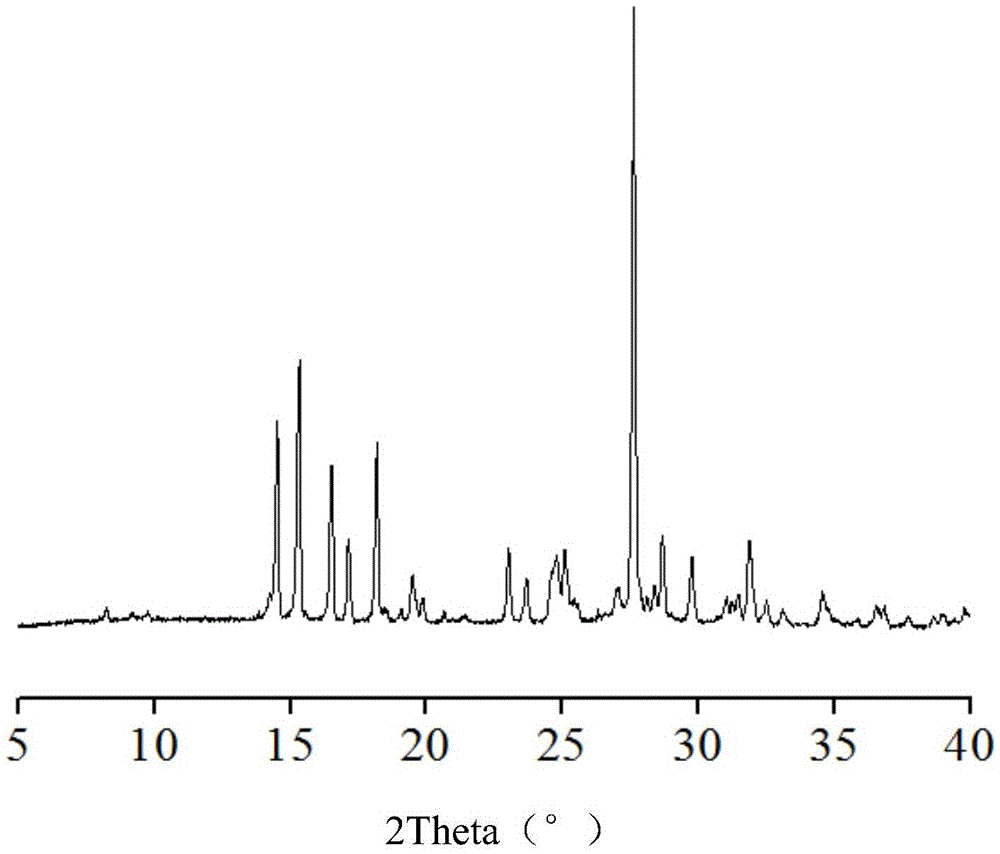
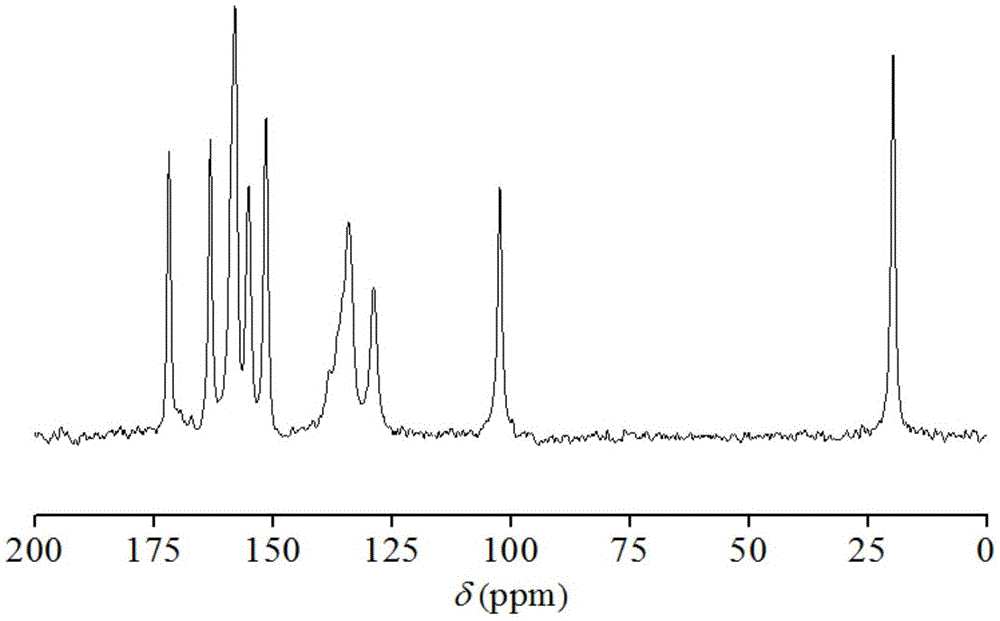
Hemi-5-fluorocytosine salt, as well as preparation method and application thereof
ActiveCN103965116AHigh effective contentHas a sweet tasteAntimycoticsOrganic chemistryDrug contentPharmaceutical industry
The invention discloses hemi-5-fluorocytosine salt, as well as a preparation method and application thereof. The salt adopts acesulfame salt, and mainly composed of 5-fluorocytosine cation (FCH+), 5-fluorocytosine neutral molecule (FC) and acesulfame anion (AH-) according to the molar ratio of 1 to 1 to 1, the crystal cell of the salt belongs to an anorthic system, has the axial length a being equal to 7.2613(2)A, b being equal to 10.5289(3)A, and c being equal to 11.6662(4)A, and has the axial angle Alpha being equal to 66.8180(11) degrees, Beta being equal to 82.0220(12) degrees, and Gamma being equal to 78.5670(12) degrees. The hemi-5-fluorocytosine salt provided by the invention can effectively solve the problem of low moisture stability of the 5-fluorocytosine,and has excellent and effective medicine content and heat stability, the preparation technology of the hemi-5-fluorocytosine salt is simple, the operation is easy, the cost is low, poisonous solvent residue is avoided, meanwhile, the object molecule component serves as a sweetener, the medical mouthfeel can be improved, and the hemi-5-fluorocytosine salt is applicable to pharmaceutical industry.
Owner:SUZHOU INST OF NANO TECH & NANO BIONICS CHINESE ACEDEMY OF SCI
Synthesis method of capecitabine
InactiveCN103193842AThe synthesis process is simpleReduce manufacturing costSugar derivativesSugar derivatives preparationPtru catalystTriflic acid
The invention relates to the technical field of synthesis of chemical medicines, and in particular relates to a synthesis method of capecitabine. The synthesis method of the capecitabine comprises the following steps: reacting 5-flucytosine with 5-deoxy-1,2,3-triacetoxy-D-ribose in the presence of a catalyst; performing aftertreatment on the reaction liquid to obtain 2',3'-diacetoxy-5'-deoxy-5-fluorocytidine; adding pentyl chloroformate dropwise into the 2',3'-diacetoxy-5'-deoxy-5-fluorocytidine and pyridine; and performing aftertreatment on the reaction liquid, adding alkaline liquid dropwise and performing treatment to obtain the capecitabine, wherein the catalyst comprises trifluoromethanesulfonic acid and derivatives thereof. The catalyst used in the method can use alpha, beta isomer mixed type 5-deoxy-triacetyl ribose as a raw material and does not need to separate ALFA and BETA isomers; and the generated ALFA type glucoside can be converted into BETA type glucoside, so the production process is simplified and the production cost is reduced.
Owner:JINING HIGH TECH DEV ZONE YONGFENG CHEM PLANT
Suitqable to industrialized method for preparing emtricitabine
ActiveCN1274687CRaw materials are easy to getHigh yieldOrganic chemistryDigestive systemHydration reactionPtru catalyst
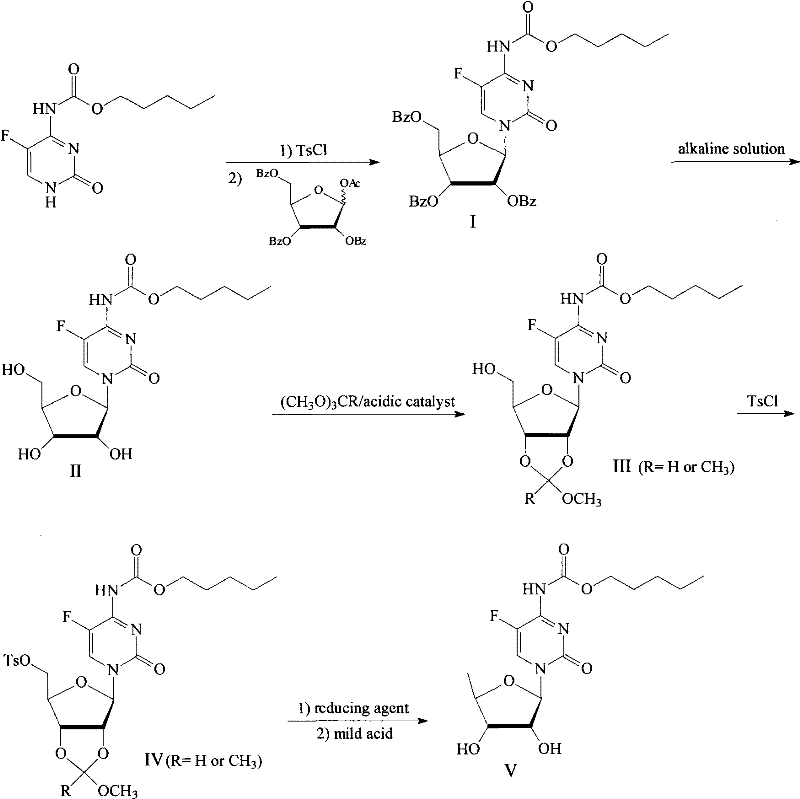
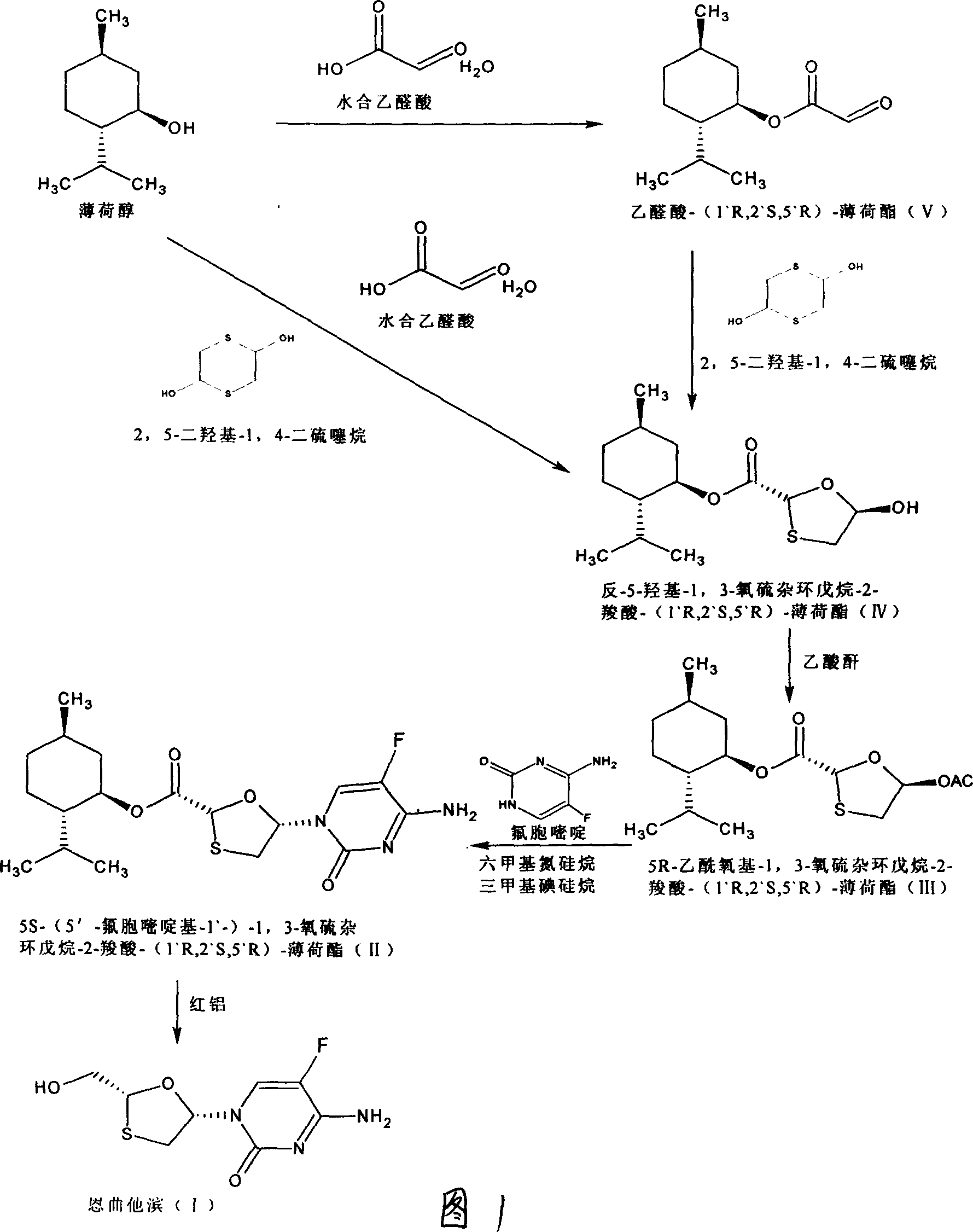
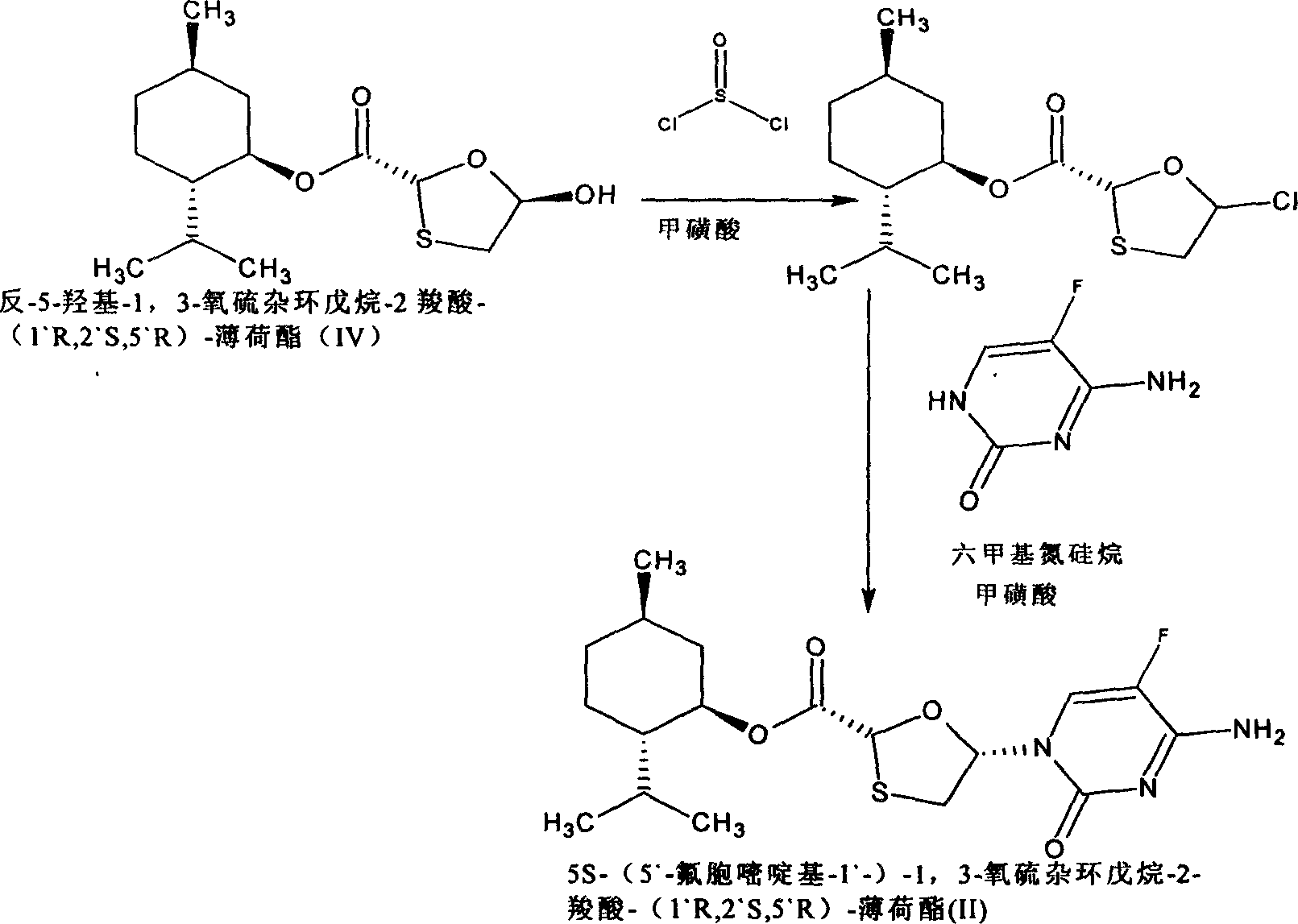
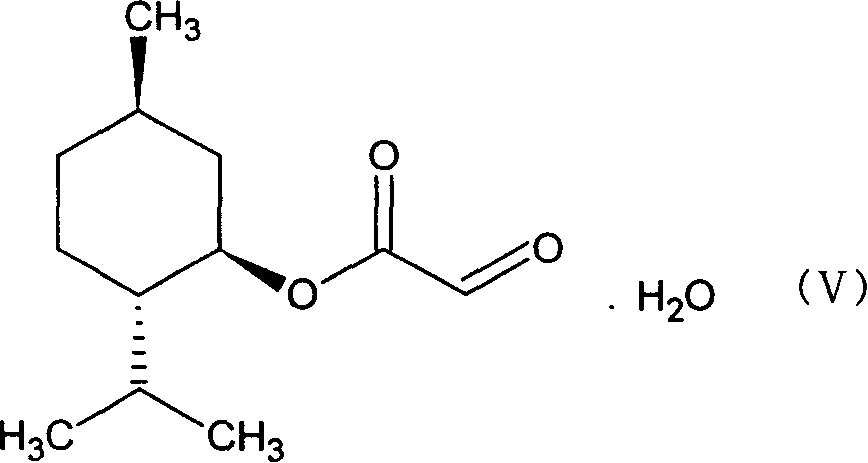
This invention prepn. method consists procedures (as shown in route chart) of: (1). hydrated glyoxalic acid is reacted with menthol in solvent and catalyst to produce stable intermediate-glyoxalic (1,R,2,5,5,R) menthol ester (V); then, reacting with 2,4-dihydroxy-1,4-dithiothiane to produce menthol ester (IV); (2). said product being proceeded acidylation by hydroxy to produce menthol ester (III); (3) being condensed with 5-fluorocytosine under the protection of silanized reagent, after purification to obtain menthol ester (II); (4). above-said product is reduce by reducing agent to obtain final invention product enqutabin (I) This invention has advantages of: available raw material, high yield, safety and commercialization prodn.
Owner:SHANDONG WEIFANG PHARMA FACTORY
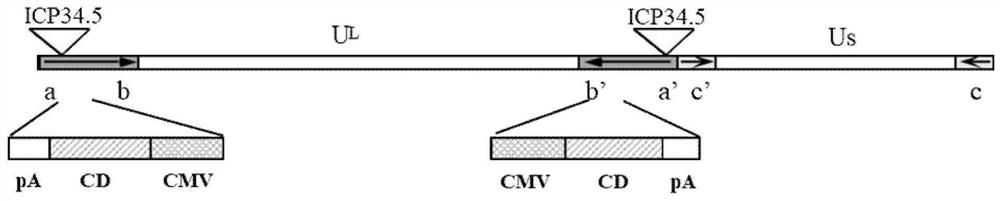
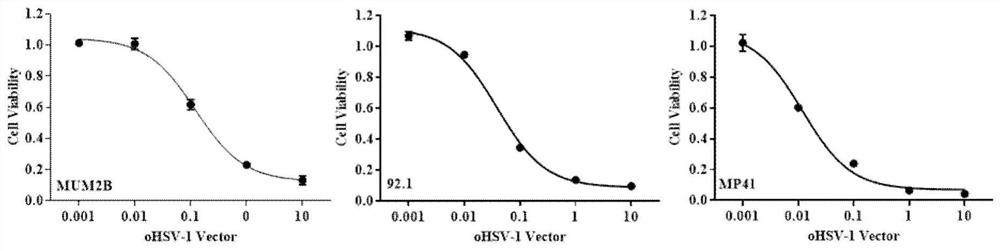
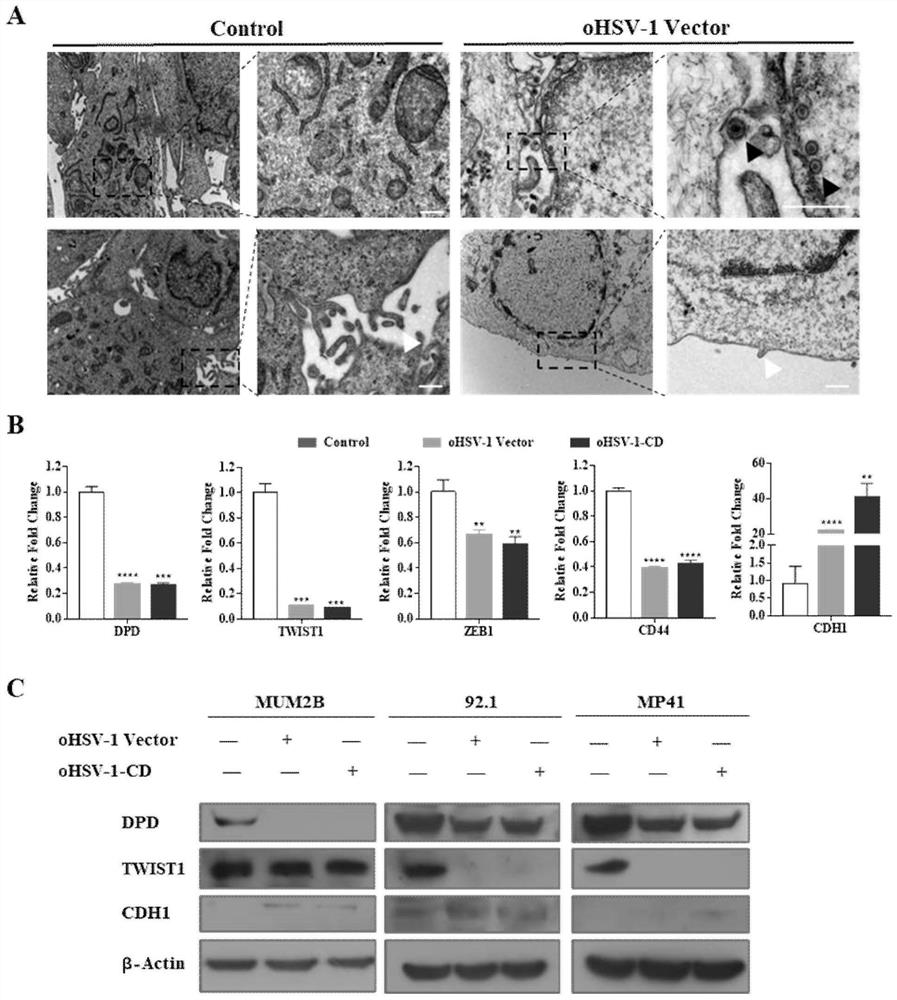
Application of oncolytic virus in treatment on uveal melanoma, marker of treatment effect and detection reagent thereof
ActiveCN111850126AEnhanced cell killingIncrease lethalityOrganic active ingredientsPeptide/protein ingredientsCytosine deaminaseCD44
The invention discloses a preparation for treating, preventing and / or slowing down uveal melanoma, the preparation comprises oncolytic herpes simplex virus type 1 capable of expressing cytosine deaminase genes and 5-fluorocytosine, and the oncolytic herpes simplex virus type 1 and the 5-fluorocytosine are combined to significantly reduce the volume of the uveal melanoma and improve the lifetime ofa subject. The invention further discloses a marker for evaluating the treatment effect of uveal melanoma through the preparation, and the marker is selected from markers capable of representing theepithelial-mesenchymal transition degree and mainly comprises IL-6, DPD, TWIST1, ZEB1, CD44 and CDH1.
Owner:BEIJING NEUROSURGICAL INST
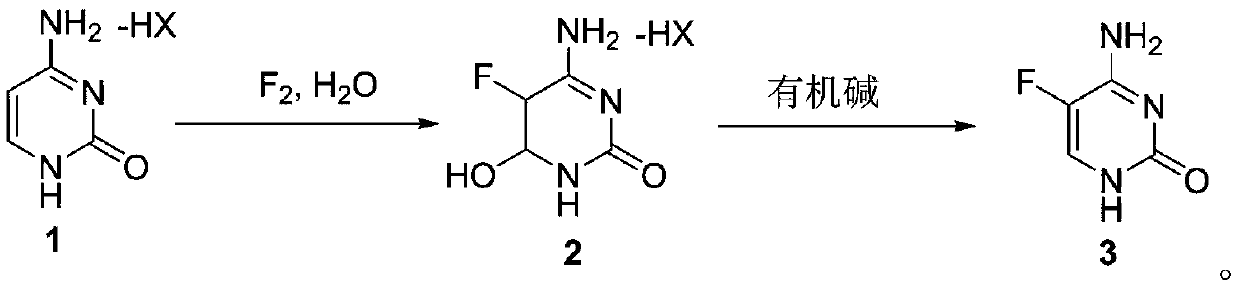
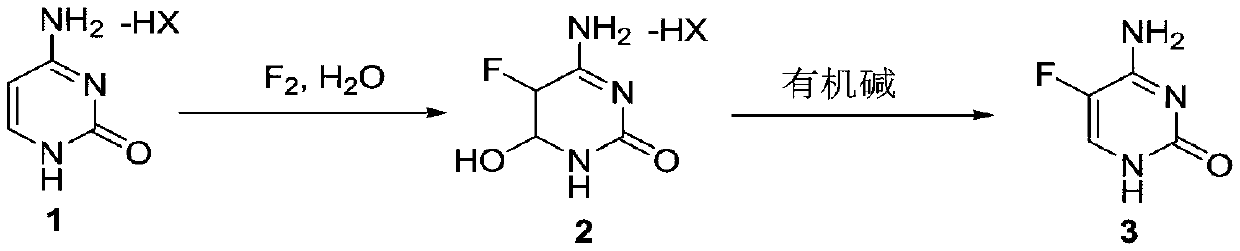
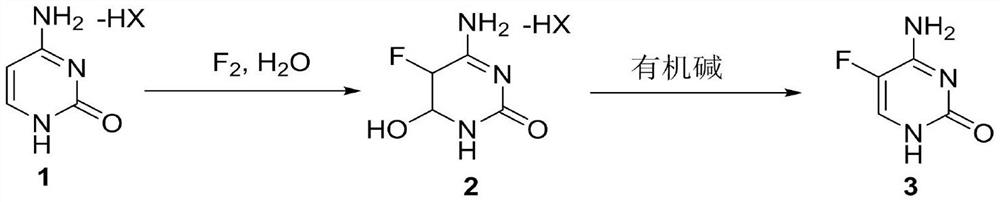
Method for synthesizing 5-flucytosine
ActiveCN110746360AImprove conversion rateImprove product qualityOrganic chemistryCytosineOrganic base
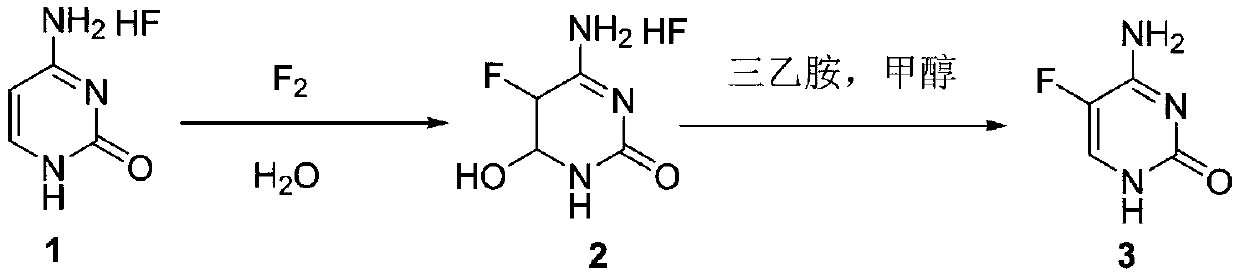
The invention discloses a method for synthesizing flucytosine and belongs to the field of nucleoside synthesis in organic chemistry. The method comprises the following reaction steps: subjecting cytosine salt 1, which serves as a raw material, to a reaction with fluorine gas by taking water as a solvent so as to obtain an intermediate 2, then, subjecting the intermediate 2 to a reaction with an organic base, carrying out dehydrating so as to obtain crude 5-flucytosine, and carrying out water refining, thereby obtaining the 5-flucytosine 3. According to the method, the operation is simple, theemploying of solvents with relatively large danger such as hydrofluoric acid is avoided, the corrosiveness to equipment is also lowered greatly, and meanwhile, the production cost is reduced, so thatthe method is relatively applicable to industrial production.
Owner:TUOXIN GROUP +1
5-fluorocytosine fluorination reactor with stirring function
PendingCN105709665AGuaranteed mass transfer effectGuaranteed leakOrganic chemistryChemical/physical processesFluid phaseElectric discharge
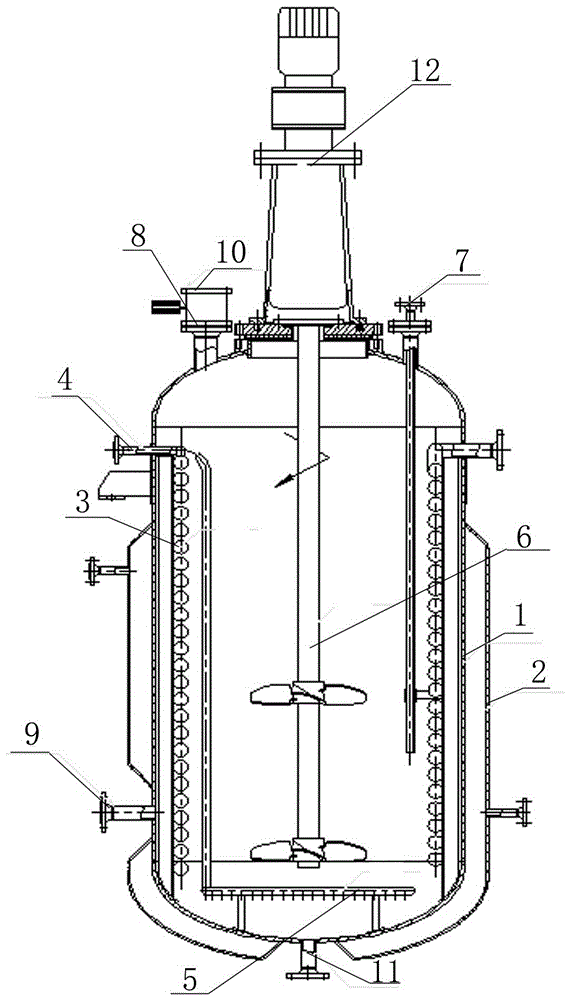
The invention discloses a 5-fluorocytosine fluorination reactor with the stirring function. The outer surface of a barrel (1) is sleeved with a cooling jacket (2); the middle of the upper portion of the barrel (1) is provided with a solid material adding device (12); one side of the upper portion of the barrel (1) is provided with a gaseous phase outlet (10) which is internally provided with an electric discharging valve (8); the other side of the upper portion of the barrel (1) is provided with a temperature measuring sleeve (7); the upper portion of the temperature measuring sleeve (7) is located outside the barrel (1); the lower portion of the temperature measuring sleeve (7) is located in the barrel (1); the upper portion of the side face of the barrel (1) is provided with a fluorine and nitrogen inlet pipe (4) which is communicated with the interior of the barrel (1); a gas distributor (5) is arranged at the bottom of the barrel (1); a cooling coil pipe (3) is arranged on the inner surface of the barrel (1); a stirrer (6) is arranged at the center of the barrel (1); a liquid level meter port (9) is formed in the side face of the lower portion of the barrel (1); a liquid phase discharge port (11) is formed in the bottom of the barrel (1).
Owner:江苏梅兰化工有限公司
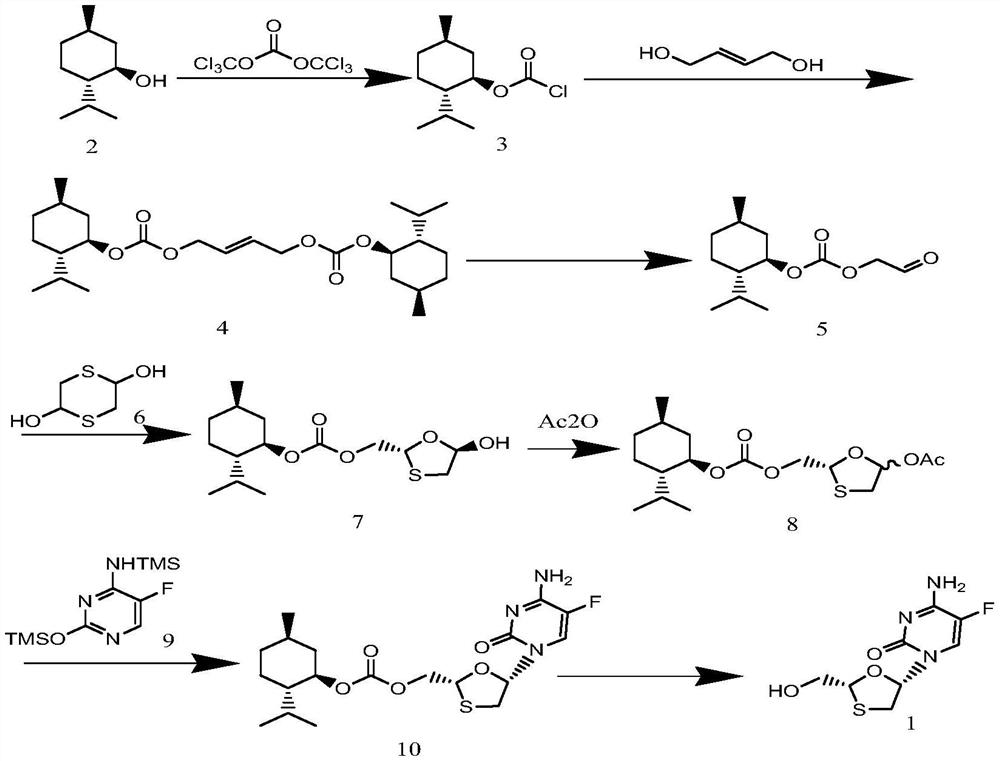
Novel process for preparing emtricitabine intermediate
InactiveCN106187988AEasy to operateLess corrosiveGroup 4/14 element organic compoundsSilanesSodium iodide
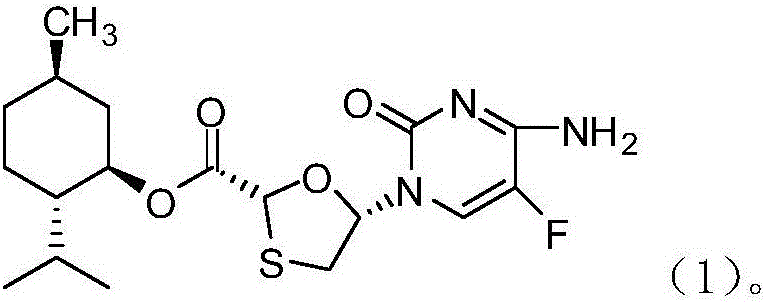
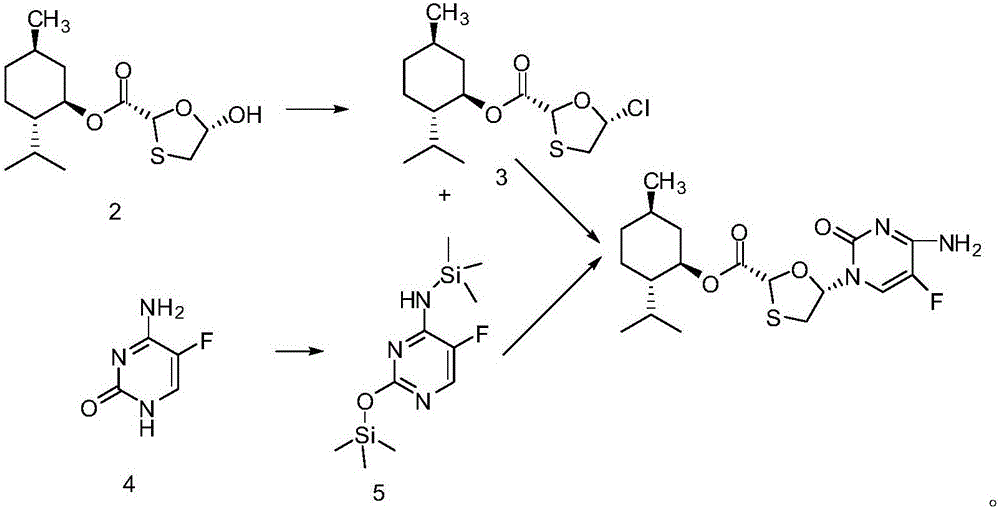
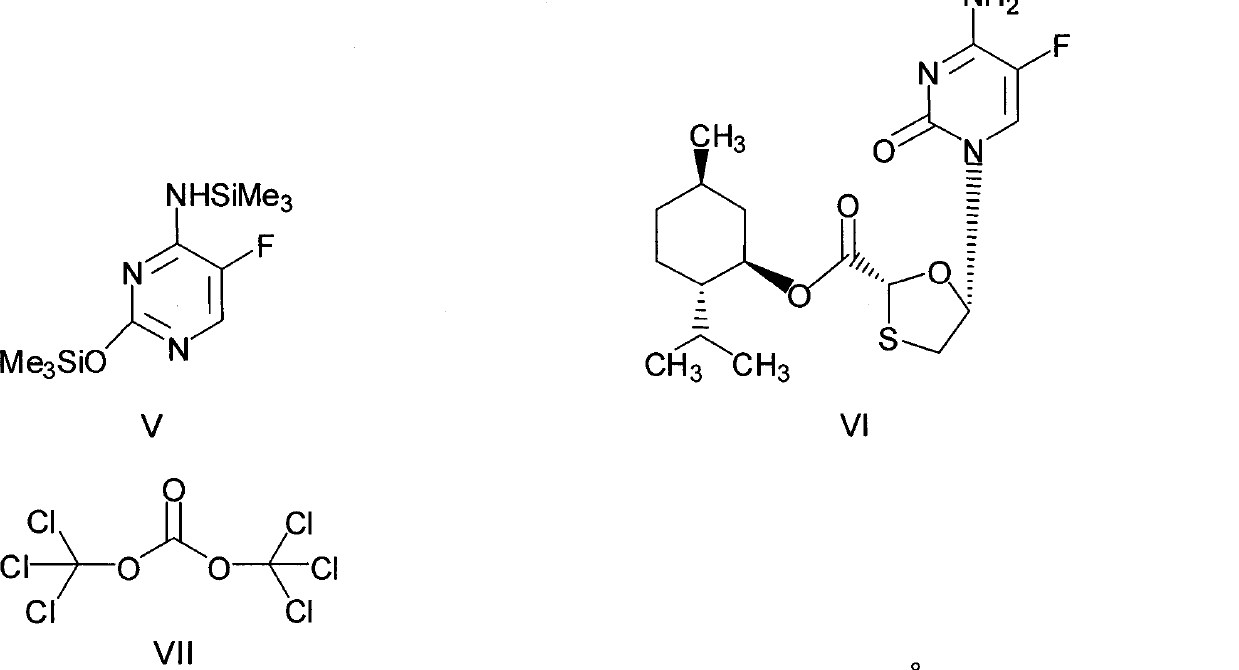
The invention provides a novel process for preparing an emtricitabine intermediate, namely, a compound shown in the formula (1) in the description, and relates to the field of medicine synthesis. The novel process includes the steps that (2R,5R)-5-hydroxy-1,3-oxathiolane-2-carboxy l-menthyl ester is subjected to triphosgene chlorination and reacts with 5-flucytosine protected with silane, and then the fine compound in the formula (1) is obtained through hydrolysis and crystallization with acetonitrile. The total yield reaches 85% or above, the purity is 99.7% or above, andany individual impurity is 0.2% or below; triphosgene replaces thionyl chloride for chlorination of (2R,5R)-5-hydroxy-1,3-oxathiolane-2-carboxy l-menthyl ester, operation is convenient, corrosivity is small, and the service life of equipment can be prolonged; sodium iodide is added into a silanization reaction of 5-flucytosine, and the yield is greatly increased.
Owner:XIAMEN CITY WEI JIA CHEM TECH CO LTD
A kind of preparation method of 2',3'-di-o-acetyl-5'-deoxy-5-fluorocytidine
ActiveCN102993253BLess impuritiesShort reaction timeSugar derivativesSugar derivatives preparationPtru catalystDeoxyribose
The invention discloses a preparation method of 2',3'-bi-O-acetyl-5'-deoxy-5-fulurocytidine. The preparation method comprises the following steps of: carrying out glycosylation reaction on 1,2,3-tri-O-acetyl-5-deoxyribose and bi-silanized 5-fluorocytosine under the catalysis of polymer-supported lewis acid which can be separated and reused for multiple times to obtain reaction liquid, and separating and purifying the reaction liquid to obtain a high-purity important capecitabine intermediate, namely the 2',3'-bi-O-acetyl-5'-deoxy-5-fulurocytidine. According to the preparation method, the catalyst is stable and efficient, can be easily separated from a reaction system and is free of pollution and single in stereoselectivity; the content of an Alpha isomer in the reaction liquid is smaller than 1.4 percent and can be removed by recrystallization; and the 2',3'-bi-O-acetyl-5'-deoxy-5-fulurocytidine has the optical purity up to 99.6 percent and is suitable for industrial production.
Owner:ZHEJIANG XIANFENG TECH
5-flucytosine salt as well as preparation method and application thereof
ActiveCN103965115AHigh crystal purityBoundary condition wideOrganic active ingredientsOrganic chemistryPharmaceutical industryPhysical chemistry
The invention discloses a 5-flucytosine salt as well as a preparation method and application thereof. The 5-flucytosine salt is an acesulfame salt, and mainly comprises 5-flucytosine cation (FCH+), acesulfame anion (AH-) and hydrone (H2O) according to the molar ratio of 1:1:0.5, wherein the structure cell belongs to a monoclinic system; the axial length a is equal to 26.3596 (11) angstroms; b is equal to 5.8523 (3) angstroms; c is equal to 32.3137 (11) angstroms; the axial angle alpha is equal to 90.00 degrees; beta is equal to 105.987 (4) degrees; gamma is equal to 90.00 degrees. The 5-flucytosine salt disclosed by the invention can effectively solve the problem that 5-flucytosine is low in humidity stability; the 5-flucytosine salt is of high heat stability, and can be prepared by conventional methods such as a lyophilization method and a solution crystallization process; the preparation method is simple in process, easy in operation, low in cost and free of poisonous solvents; in the meantime, as a sweetening agent, the guest molecule can improve the taste of a drug, and can be applied to the pharmaceutical industry.
Owner:SUZHOU INST OF NANO TECH & NANO BIONICS CHINESE ACEDEMY OF SCI
Method for preparing capecitabine intermediate 2', 3'-di-O-acetyl-5'-deoxy-5-fluorocytidine by enzymatic composite chemical method
The invention provides a preparation method of a capecitabine intermediate 2', 3'-di-O-acetyl-5'-deoxy-5-fluorocytidine, which is suitable for actual industrial mass production and has the advantages of relatively high yield, quality and good stability. The preparation method of the capecitabine intermediate comprises the following steps: creatively converting substrates 5-fluorocytosine and 5-deoxyribose-1-phosphate to 5'-deoxy-5-fluoro-cytidine by using an enzyme catalyst pyrimidine nucleoside phosphorylase (EC2.4.2.2), and further performing acetylation reaction on the 5'-deoxy-5-fluoro-cytidine to obtain the 2', 3'-di-O-acetyl-5'-deoxy-5-fluorocytidine. The method has the advantages of high yield and good quality and is in line with environmental protection requirements; the process is simple and easy to operate.
Owner:北京六盛合医药科技有限公司
Capecitabine intermediate
ActiveCN111377988AHigh yieldHigh puritySugar derivativesOrganic chemistry methodsPtru catalystRibose
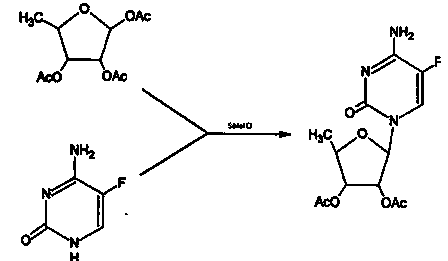
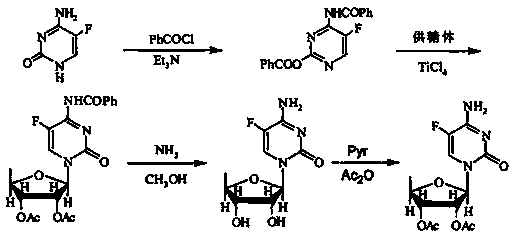
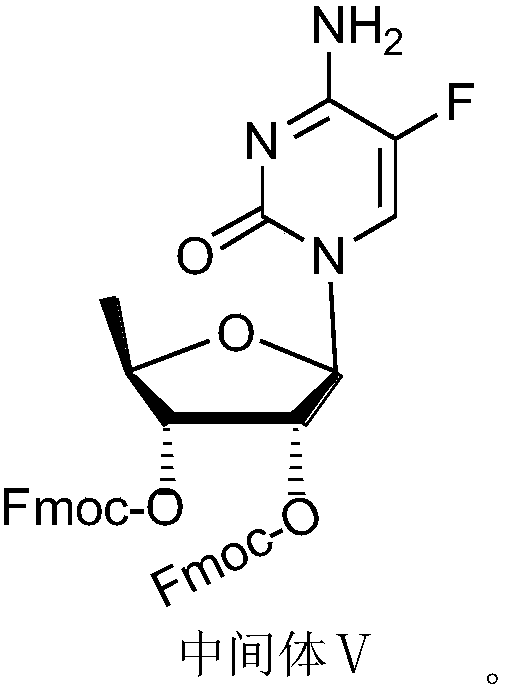
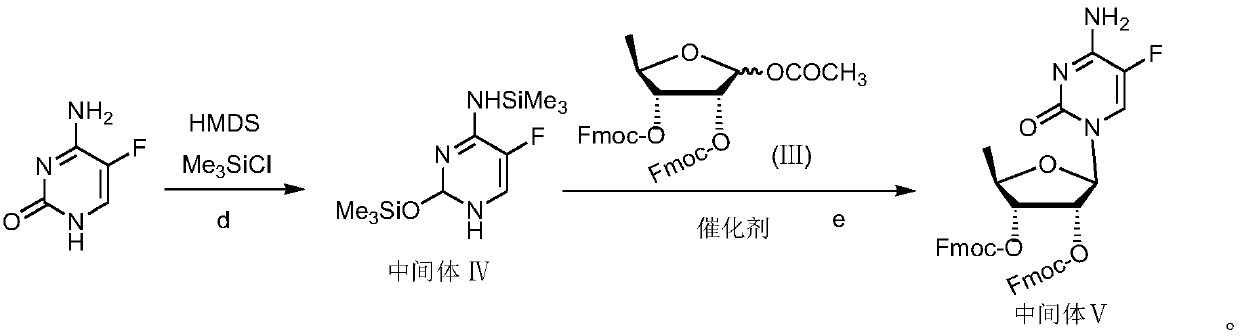
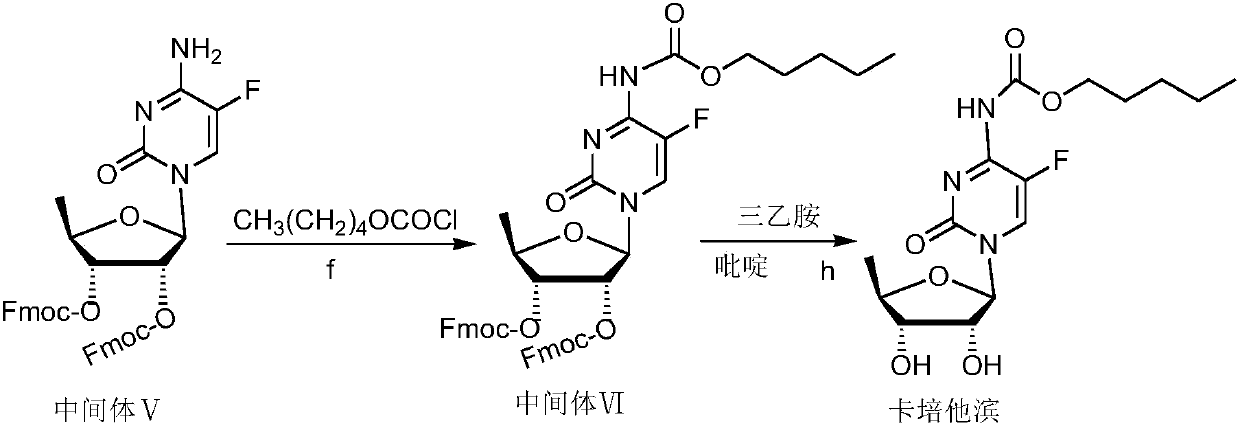
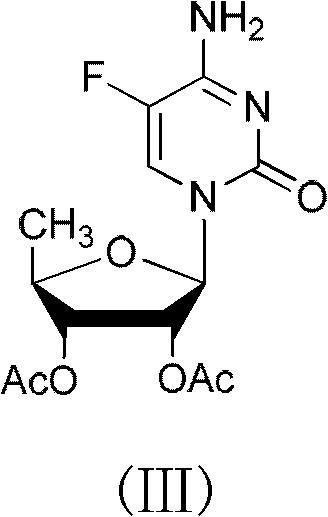
The invention belongs to the field of medicine synthesis, and discloses a capecitabine intermediate (V) and a preparation method thereof. The preparation method comprises the step of under the actionof a catalyst, enabling derivatives (III) of 5-deoxy-D-ribose of which 2,3-hydroxyl is protected with Fmoc- and activated 5-flucytosine (IV) to be coupled to obtain the capecitabine intermediate (V).The method is simple and easy to operate, simple in post-treatment, high in product purity and suitable for industrial production.
Owner:LUNAN PHARMA GROUP CORPORATION
2',3'- di-O-acetyl-5'-deoxy-5-fulurocytidineonium compound and preparation method thereof
ActiveCN102424697AReduce usageClear structureSugar derivativesSugar derivatives preparationCombinatorial chemistryBiological activation
The invention relates to a 2',3'- di-O-acetyl-5'-deoxy-5-fulurocytidineonium compound of formula (I) or a solvate thereof. The compound is prepared by using 5-deoxy-1,2,3-tri-O-acetyl-beta-D-ribofuranose as a raw material, carrying out condensation reaction on 5-deoxy-1,2,3-tri-O-acetyl-beta-D-ribofuranose and protected 5-flucytosine under the activation of iodotrimethylsilane to obtain a reaction product, and carrying out deprotection, acidification and crystallization. According to the invention, a novel intermediate for synthesizing Capecitabine is developed, one pot process is adopted for reacting to obtain the 2',3'- di-O-acetyl-5'-deoxy-5-fulurocytidineonium compound, and high-purity Capecitabine can be further synthesized by using the 2',3'- di-O-acetyl-5'-deoxy-5-fulurocytidineonium compound as a key intermediate. The compound has the characteristics of clear structure, high purity and stable quality. The use of the compound to synthesize Capecitabine can reduce reaction steps, control process cost, and reduce environmental pollution.
Owner:山东安信制药有限公司
Method for synthesizing emtricitabine intermediate
ActiveCN101391997BOperational securityThe reaction steps are simpleOrganic chemistryEmtricitabineCarboxylic acid
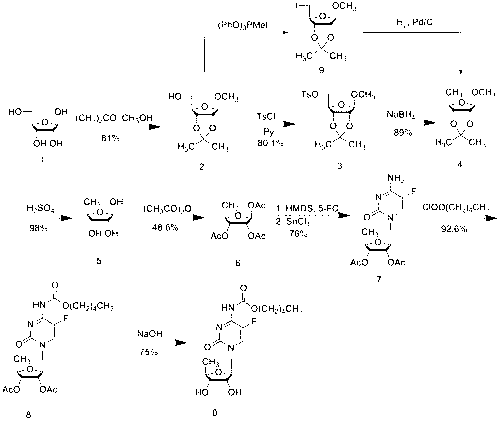
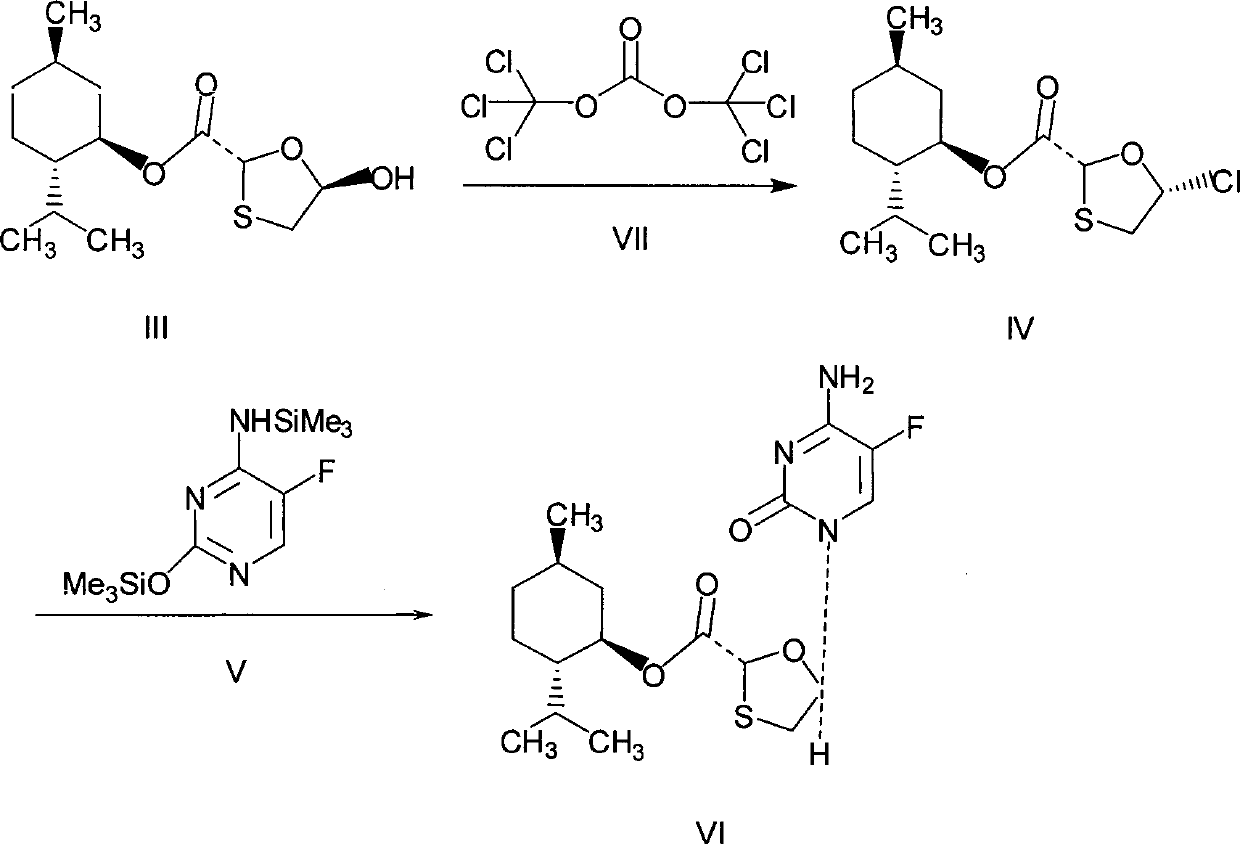
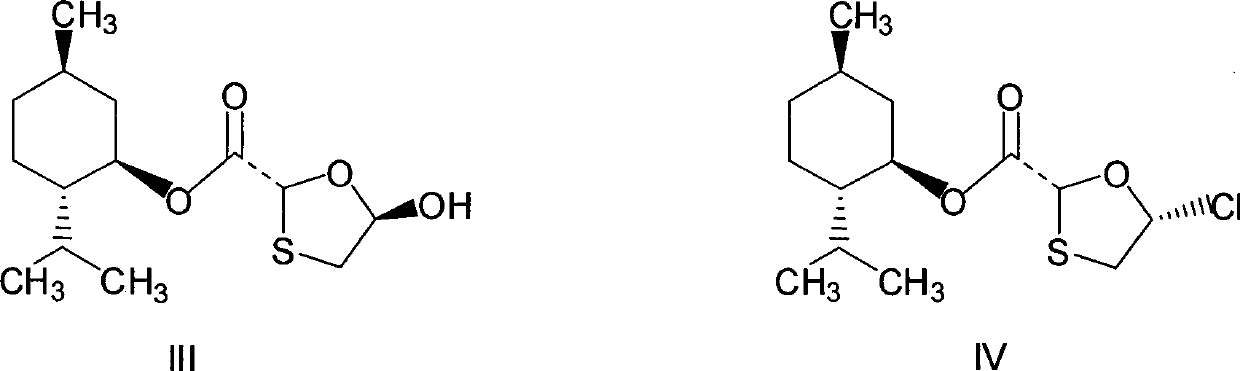
The invention discloses a synthetic method of emtricitabine intermediates, namely, (2R, 5S)-5-(5'-fluoro-cytimidine-1-group)-1, 3-oxathiolane-2-carboxylic acid-L-menthyl ester having the structural formula of (VI), (2R, 5S)-5-hydroxy-1, 3-oxathiolane-2-carboxylic acid-L-menthyl ester having the structural formula of (III) is taken as raw material to obtain chloro compounds having the structural formula of (IV) by chlorination reaction, then the chloro compounds (IV) are treated with condensation and hydrolyzation reactions with N, O-bis(trimethoxy)5-flurocytosin having the structural formula of (V), thus obtaining the (2R, 5S)-5-(5'-fluoro-cytimidine-1-group)-1, 3-oxathiolane-2-carboxylic acid-L-menthyl ester (VI). The invention uses bis(trichloromethyl) carbonic ester to replace the thionyl chloride in the prior art so as to be taken as a chlorination reagent, thus having the advantages of safe and reliable operation and environmental protection.
Owner:JIANGSU COBEN PHARMA CO LTD +1
Preparation method of capecitabine intermediate suitable for industrial production
PendingCN113321693AReduce processing costsHigh yieldSugar derivativesSugar derivatives preparationSodium bicarbonateDistillation
The invention discloses a preparation method of a capecitabine intermediate suitable for industrial production. The preparation method of the capecitabine intermediate comprises the following steps: carrying out feeding reaction, namely adding acetonitrile, 5-flucytosine, hexamethyldisilazane, trifluoromethanesulfonic acid, 1,2,3-triacetoxy-5-deoxy-D-ribose and trifluoromethanesulfonic acid into a reaction kettle, and performing stirring; carrying out concentrating reaction, namely performing reduced pressure distillation on the solution in the reaction kettle until the volume is 4 times of the reference volume, and adding dichloromethane which is 5-6 times of the reference volume and a sodium bicarbonate solution with the concentration of 8.9 mol / L; carrying out liquid separation and extraction to obtain an aqueous phase and an organic phase; carrying out equal-liquid-level distillation, namely distilling the organic phase until the volume of the liquid is 2.5 times of the volume of the starting material, maintaining the liquid level by continuously replenishing isopropanol in the subsequent distillation process, and replenishing isopropanol to the distillation end-point liquid level after dichloromethane in the system is removed; and then, carrying outcrystallization and filtration drying to obtain the capecitabine intermediate. According to the method, conventional distillation replacement is replaced by equal-liquid-level distillation operation on time; and moreover, the preparation route is further optimized. Thus, the method is suitable for industrial production and popularization of capecitabine intermediates.
Owner:SCINOPHARM CHANGSHU PHARMA
Process for Producing Fluorocytosine and Fluorocytosine Derivatives
The present invention relates to a method of manufacturing a fluorocytosine-based compound of Formula I.The invention also relates to a compound obtained by such a method, a pharmaceutical drug substance and a method for its manufacture, a pharmaceutical composition, and also various uses in therapy of the compounds, pharmaceutical drug substances, and pharmaceutical compositions of the invention.
Owner:UNIVERSITY OF DURHAM
A kind of 5-fluorocytosine salt, its preparation method and application
ActiveCN103965115BHigh crystal purityBoundary condition wideOrganic active ingredientsOrganic chemistryPharmaceutical industryPhysical chemistry
The invention discloses a 5-fluorocytosine salt, its preparation method and application. The 5-fluorocytosine salt is acesulfame potassium salt, which is mainly composed of 5-fluorocytosine cation (FCH+), acesulfame potassium anion (AH-) and water molecule (H2O) in a molar ratio of 1:1:0.5 , its unit cell belongs to the monoclinic system, its axial length a=26.3596(11)? , b=5.8523(3)? , c=32.3137(11)? , axis angle α=90.00°, β=105.987(4)o, γ=90.00o. The 5-fluorocytosine salt of the present invention can effectively improve the problem of poor moisture stability of 5-fluorocytosine, and has good thermal stability, and can be prepared by conventional methods such as freeze-drying method and solution crystallization method, with simple process and easy operation Yes, low cost, no toxic solvents involved, and at the same time, the guest molecule is a sweetener, which can improve the taste of medicines, and can be used in the pharmaceutical industry.
Owner:SUZHOU INST OF NANO TECH & NANO BIONICS CHINESE ACEDEMY OF SCI
Half 5-fluorocytosine salt, its preparation method and application
ActiveCN103965116BHigh effective contentHas a sweet tasteAntimycoticsOrganic chemistryDrug contentPharmaceutical industry
The invention discloses a semi-5-fluorocytosine salt, its preparation method and application. The salt is acesulfame potassium salt, which is mainly composed of 5-fluorocytosine cation (FCH+), 5-fluorocytosine neutral molecule (FC) and acesulfame potassium anion (AH-) in a molar ratio of 1:1:1 Composition, and the unit cell of the salt belongs to the triclinic system, its axial length a=7.2613 (2)? , b=10.5289(3)? , c=11.6662(4)? , axis angle α=66.8180(11)°, β=82.0220(12)o, γ=78.5670(12)o. The semi-5-fluorocytosine salt prepared by the invention can effectively improve the problem of poor moisture stability of 5-fluorocytosine, has good effective drug content and thermal stability, and has simple preparation process, easy operation and low cost , there is no toxic solvent residue, and the guest molecular component is a sweetener, which can improve the taste of the drug and is suitable for the pharmaceutical industry.
Owner:SUZHOU INST OF NANO TECH & NANO BIONICS CHINESE ACEDEMY OF SCI
A kind of preparation method of emtricitabine
ActiveCN109438432BStarting materials are cheap and readily availableMild reaction conditionsOrganic chemistry methodsMentholEmtricitabine
The invention discloses a preparation method of emtricitabine. Purified to obtain pure 5S-(5'-fluorocytosinyl-1')-1,3-oxathiolane-2-ethoxycarbonyl-(1'R, 2'S, 3'R)-menthyl ester ; Slough the chiral auxiliary agent L Menthol under the condition of weak base and solvent to obtain product emtricitabine. The starting materials required by the present invention are cheap and easy to obtain, the reaction conditions are mild, the utilization rate of atoms is high, the operation process is simple, the reagents used are green and environmentally friendly, the obtained product has high chemical purity, reaches the pharmaceutical standard, and is suitable for industrial production of emtricitabine .
Owner:WUHAN INSTITUTE OF TECHNOLOGY
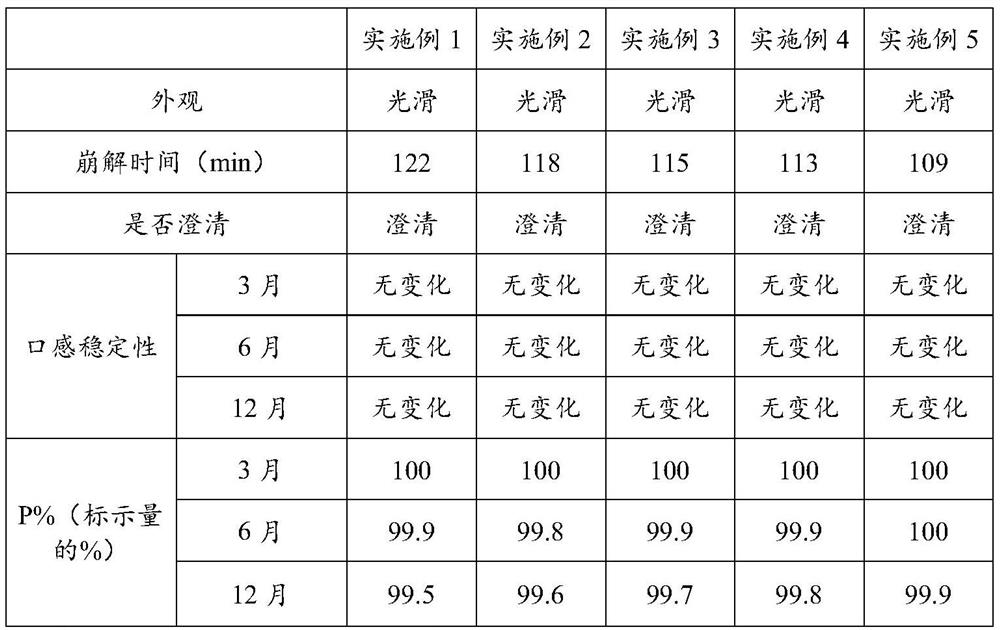
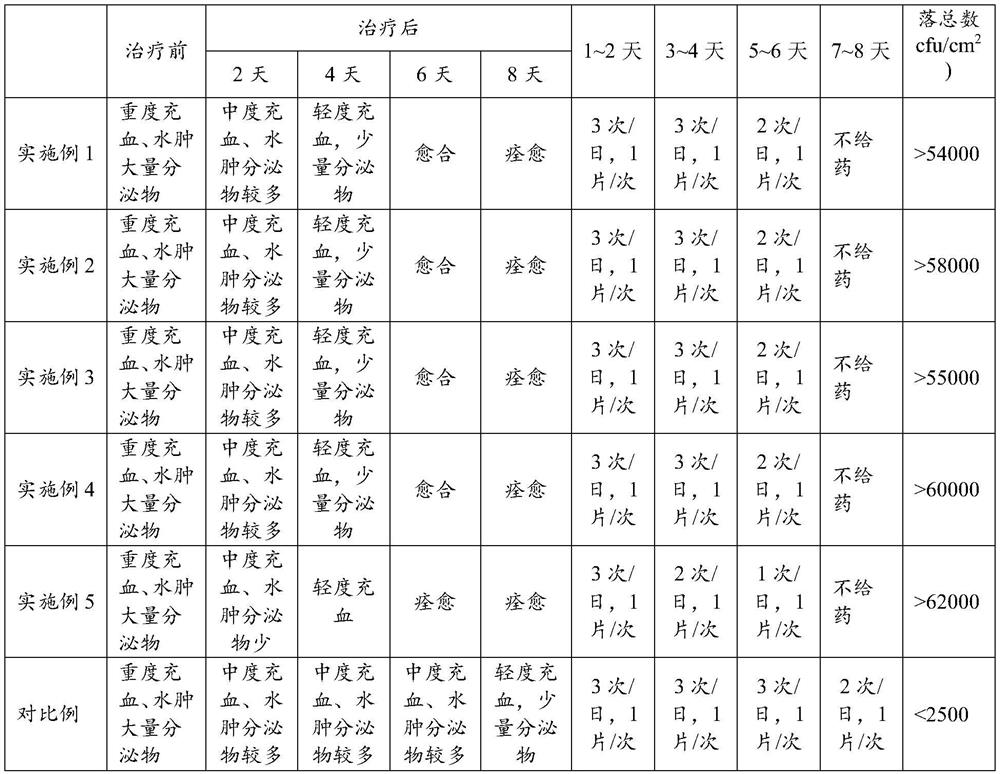
Fluorocytosine tablet and preparation method thereof
ActiveCN113081987ADelay drug resistanceGood treatment effectOrganic active ingredientsAntimycoticsBiotechnologyCellulose
The invention discloses a flucytosine tablet and a preparation method thereof, and the flucytosine tablet comprises the following components: 31.2-67.8 parts of flucytosine, 4.7-13.2 parts of aztreonam, 20-30 parts of maltodextrin, 14-18 parts of pea meal, 10-15 parts of rice bran, 6-9 parts of beer yeast, 3-6 parts of lactulose, 3-6 parts of a compound probiotic agent, 9-16 parts of sodium carboxymethyl starch, and 3-7 parts of hydroxypropyl methyl cellulose, wherein in each flucytosine tablet, the content of flucytosine is 31.0%-36.0%, and the content of aztreonam is 4.5%-7.0%. The flucytosine tablet can improve the drug resistance of fungi due to long-term administration of flucytosine, reduce the administration frequency and reduce the administration dosage, the preparation method is simple and reliable, the prepared tablet has a smooth surface, the drug quality and the drug effect stability are effectively ensured, and the drug can be quickly dispersed, dissolved out and absorbed in gastrointestinal tracts.
Owner:药大制药有限公司
A kind of microchannel reactor, device and preparation method of 5-fluorocytosine
ActiveCN107670603BSimple structureEasy to assemble and disassembleOrganic chemistryChemical/physical/physico-chemical microreactorsMicroreactorTrifluoroacetic acid
Owner:FUJIAN YONGJING TECH CO LTD
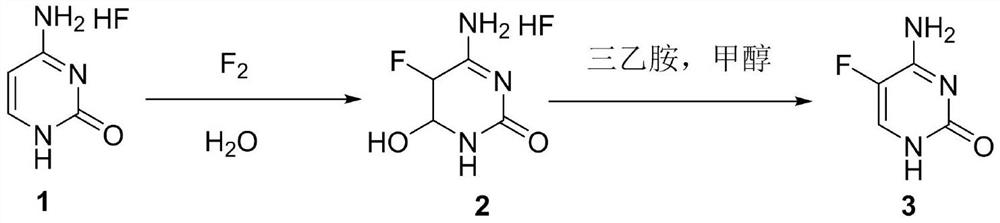
A method for synthesizing 5-fluorocytosine
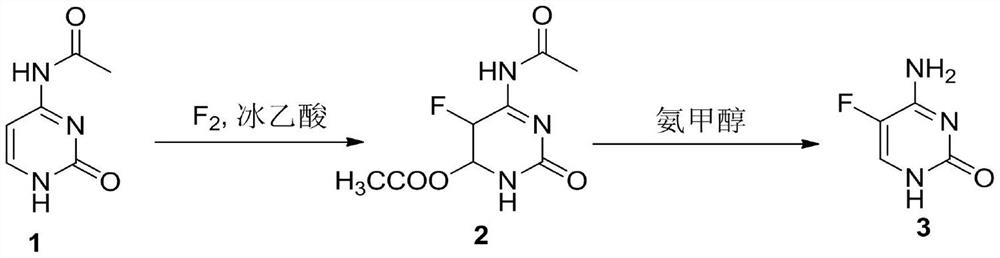
The invention discloses a method for synthesizing flucytosine, which belongs to the field of nucleoside synthesis in organic chemistry. The reaction steps are as follows: use cytosine salt 1 as raw material, use water as solvent, react with fluorine gas to obtain intermediate 2, then react with organic base, obtain 5-fluorocytosine crude product after dehydration, and obtain 5‑fluorocytosine3. The method is simple to operate, avoids the use of dangerous solvents such as hydrofluoric acid, greatly reduces the corrosion to equipment, and reduces production costs at the same time, and is more suitable for industrial production.
Owner:TUOXIN GROUP +1
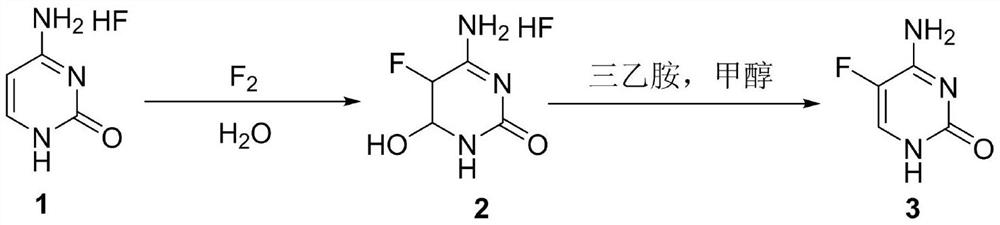
A method for synthesizing 5-fluorocytosine
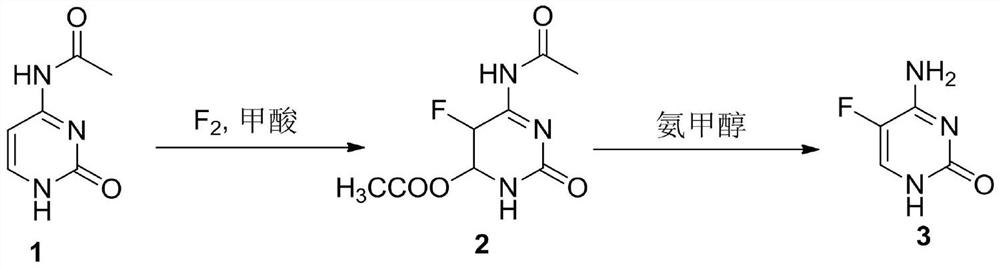
The invention discloses a method for synthesizing flucytosine, which belongs to the field of nucleoside synthesis in organic chemistry. Its reaction steps are as follows: adopt N 4 ‑Acyl-protected cytosine is used as raw material, organic carboxylic acid is used as solvent, one-step intermediate is obtained through direct fluorination, and 5‑fluorocytosine is obtained through removal reaction. The invention not only reduces the cost of raw materials, but also solves the equipment corrosion problem caused by the cytosine and hydrogen fluoride process, and at the same time, the purity of the 5-fluorocytosine obtained by the method can reach more than 99.9%.
Owner:TUOXIN GROUP +1
A detection method of 5-fluorocytosine and 5-fluorouracil in cells based on capillary electrophoresis
InactiveCN107228895BReduce dosageLow costMaterial analysis by electric/magnetic meansColor/spectral properties measurementsElectrophoresesCytosine
The invention relates to a method for detecting intracellular 5-flucytosine and 5-fluorouracil based on capillary electrophoresis. The method comprises the following steps: transfecting cells with a plasmid carrying a suicide gene FCY1, adding 5-flucytosine and / or 5-fluorouracil into a medium, and carrying out culture; then collecting cell metabolite extract; and carrying out qualitative and quantitative detection of 5-flucytosine and / or 5-fluorouracil in the cell metabolite extract by using capillary electrophoresis. The method provided by the invention has the characteristics of high separation efficiency, low sample usage amount, short separation time, low analysis cost and the like, and realizes qualitative and quantitative detection of intracellular 5-flucytosine and 5-fluorouracil.
Owner:DONGHUA UNIV
A kind of preparation method of 5-fluorocytosine
ActiveCN110105290BReduce manufacturing costReduce corrosionOrganic chemistryOrganic baseOrganic layer
The invention provides a preparation method of 5-fluorocytosine. The method is characterized by including the following steps that 1, an organic solvent, fluoroacetonitrile, ethyl formate and organicbase are added into an autoclave for a heating reaction in a predetermined gaseous environment, then decompressing and cooling are conducted, and first reaction liquid containing an intermediate 1 isobtained; 2, an alcohol solution of hydrogen chloride is added into a reaction still, the first reaction liquid is added for reaction after cooling, a second reaction liquid is obtained after reaction, water is added into the second reaction liquid, the pH value is adjusted to be 6-8, a first organic layer is obtained after standing, and the first organic layer is distilled to obtain an intermediate 2; 3, the intermediate 2 and urea are subjected to an aldimine condensation reaction, and then a crude product of 5-fluorocytosine is obtained. According to the method, the synthesis process is simple, there are a few required reaction steps, the total yield is relatively high, the safety of reaction is high, the production efficiency is improved, and the production cost is reduced.
Owner:浙江伟锋药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com