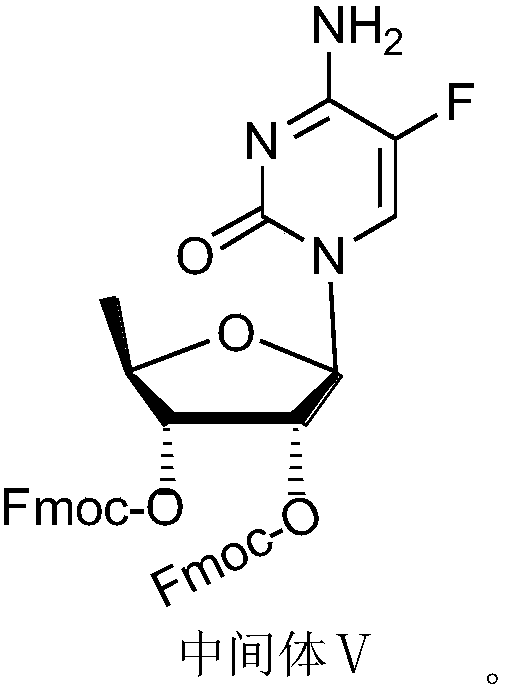
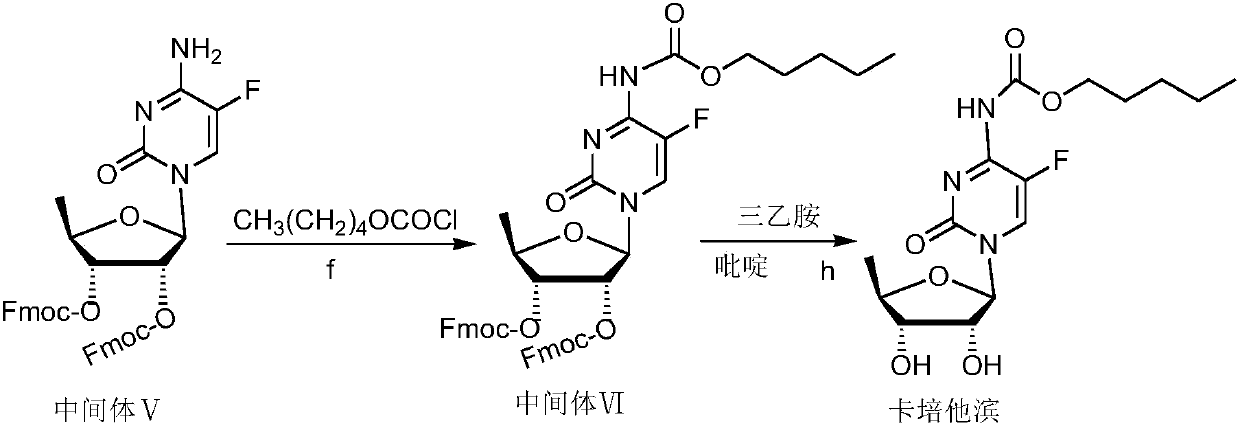
Capecitabine intermediate
A technology for capecitabine and intermediates, which is applied in the field of capecitabine intermediates and preparations, and can solve the problems of unstable 5-fluorocytosine protective agent, high cost, and low conversion rate of 5-fluorocytosine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
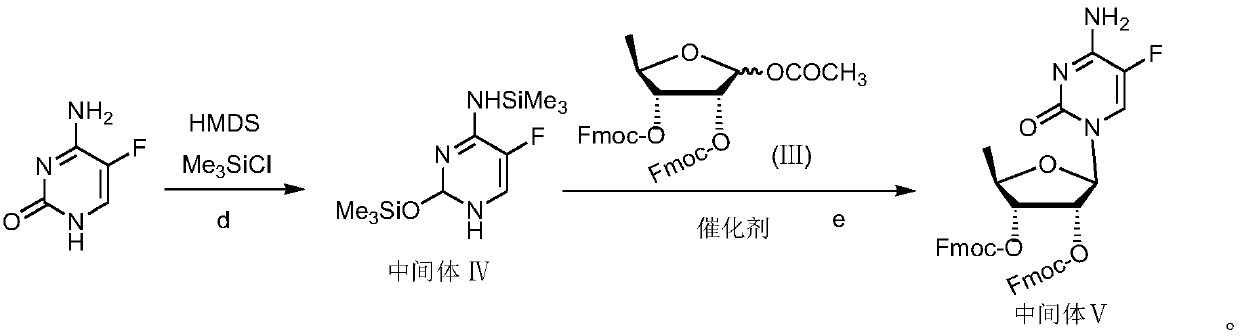
[0071] Synthesis of 5-Fluorocytosine Ⅳ Activated by Hexamethyldisilazane
[0072] In a 3000mL three-neck flask, add 258g (2mol) of 5-fluorocytosine, 728mL of toluene, 421g (2.6mol) of HMDS, 14.3g (0.13mol) of trimethylchlorosilane, stir and heat up to 100°C, and react 3-4 After the reaction was completed, the solvent was evaporated under reduced pressure to dryness to obtain white solid particles with a yield of 90%. used directly in the next reaction.
[0073] Synthesis of Capecitabine Intermediate Ⅴ
[0074] Under nitrogen protection, add 620 g (1 mol) of 5-deoxy-D-ribose derivative (Ⅲ) and 357.5 g of hexamethyldisilazane-activated 5-azacytosine IV in a 5000 mL three-necked flask (1.3mol) of the acetonitrile suspension prepared, then add 1000mL of acetonitrile, under stirring, the reaction system is kept at 5 ℃, add dropwise a solution of 200gTMSOTf (0.9mol) and 400mL of acetonitrile, TLC detection of the reaction process; after the reaction, add 1.5L chloroform, wash twi...
Embodiment 2
[0076] Synthesis of 5-Fluorocytosine Ⅳ Activated by Hexamethyldisilazane
[0077] Preparation reaction is with embodiment 1
[0078] Synthesis of Capecitabine Intermediate Ⅴ
[0079] Under nitrogen protection, add 620 g (1 mol) of 5-deoxy-D-ribose derivative (Ⅲ) and 412.5 g of hexamethyldisilazane-activated 5-azacytosine IV in a 5000 mL three-necked flask (1.5mol) of the acetonitrile suspension prepared, then add 1000mL of acetonitrile, under stirring, the reaction system is kept at 0 ℃, dropwise add a solution of 288.6gTMSOTf (1.3mol) and 500mL of acetonitrile, TLC detection of the reaction process; after the reaction, add to the reaction solution Add 1.5 L of chloroform, wash twice with 2 L of water; adjust the pH to 7.5 with saturated sodium bicarbonate solution, let stand, and separate the liquids; wash the organic phase with 2 L of saturated saline; dry the organic phase with anhydrous sodium sulfate for 3 to 4 hours ; Filtrate, evaporate the solvent under reduced press...
Embodiment 3
[0081] Synthesis of 5-Fluorocytosine Ⅳ Activated by Hexamethyldisilazane
[0082] Preparation reaction is with embodiment 1
[0083] Synthesis of Capecitabine Intermediate Ⅴ
[0084] Under nitrogen protection, in a 5000mL three-necked flask, add 620g (1mol) of 5-deoxy-D-ribose derivative (Ⅲ) and 385g (1.4 mol) of acetonitrile suspension prepared by adding 1000mL of acetonitrile, under stirring, the reaction system was kept at 5°C, and a solution of 222.2gTMSOTf (1.0mol) and 500mL of acetonitrile was added dropwise, and the reaction progress was detected by TLC; after the reaction was completed, 1.5 L chloroform, wash twice with 2L water; adjust the pH to 7.5 with saturated sodium bicarbonate solution, let stand, and separate the liquid; wash the organic phase with 2L saturated brine; dry the organic phase with anhydrous sodium sulfate for 3 to 4 hours; filter , the solvent was evaporated under reduced pressure to obtain a pale yellow foamy solid, which was dissolved by addin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com