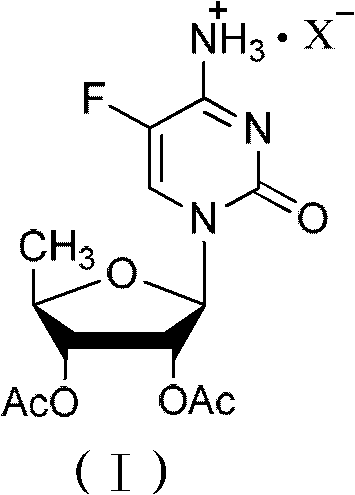
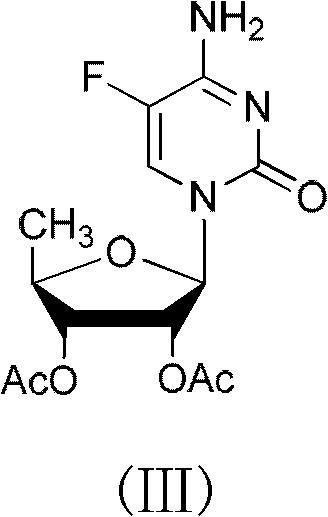
2',3'- di-O-acetyl-5'-deoxy-5-fulurocytidineonium compound and preparation method thereof
A technology of flucytidine salt and acetyl group, which is applied in the field of medicinal chemistry, can solve problems such as poor product quality, harsh reaction conditions, and cumbersome operation processes, and achieve the effects of controlling process costs, reducing reaction steps, and reducing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Synthesis of 2',3'-di-O-acetyl-5'-deoxy-5-fluorocytidine hydrochloride
[0043]
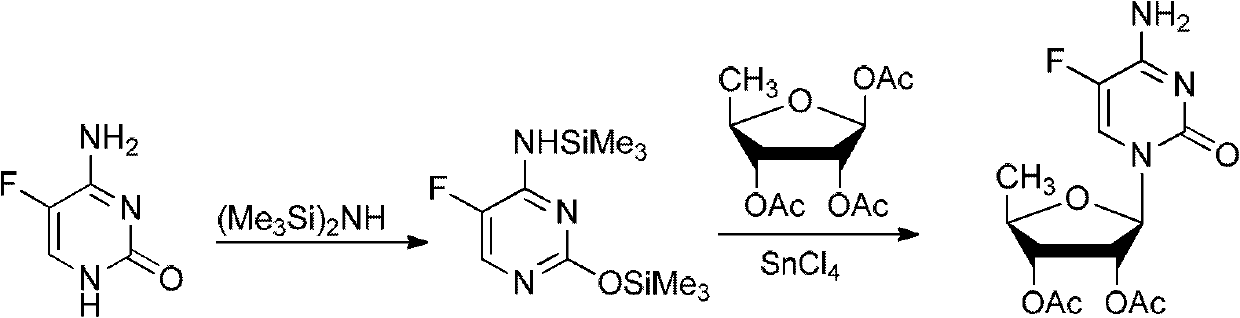
[0044] (1), the protection reaction of 5-fluorocytosine
[0045] In a dry 3L glass reaction bottle, connect the tail gas (ammonia) absorption device and the reflux tube for standby, add 300ml of dichloromethane, 100g (0.775mol) of 5-fluorocytosine, 140ml of hexamethyldisilazane, 0.5 ml iodotrimethylsilane, heat up to 70-80°C, reflux for 10-15 hours, and the liquid dissolves.
[0046] (2) Synthesis of iodide
[0047] In another reaction flask, put 500ml of dichloromethane, 201g of 5-deoxy-1,2,3-tri-O-acetyl-β-D-ribofuranose, lower the temperature to 0°C, and add 145ml of iodotrimethylsilane , keep warm at 0°C for 5 hours.
[0048] (3) Add the protected 5-fluorocytosine reaction solution into the iodide reaction solution, and react at room temperature for 3-5 hours. Then, 60 ml of isopropanol was added to the feed liquid, and a yellow-white solid was precipitated. Then, 380 ml of 10%...
Embodiment 2
[0052] Synthesis of 2',3'-di-O-acetyl-5'-deoxy-5-fluorocytidine hydrobromide
[0053]
[0054] (1) In a 2L glass reaction bottle, add 200ml of dichloromethane, add 52g of 5-fluorocytosine and 100ml of N,O-bis(trimethylsilyl)acetamide under stirring, heat up to 30-40°C, React for 3 to 5 hours, and the feed liquid is completely dissolved.
[0055] (2) In another reaction flask, put 300ml of dichloromethane, 104g of 5-deoxy-1,2,3-tri-O-acetyl-β-D-ribofuranose, cool down to 0°C, add trimethyl 85ml of iodosilane, heat at 0-5°C and react for 3-5 hours.
[0056] (3) Add the protected 5-fluorocytosine reaction solution into the iodide reaction solution, and react at room temperature for 3-6 hours. Then 50 ml of methanol was added to the feed liquid, and a yellow-white solid was precipitated. Add 300ml of 20% hydrobromic acid to the feed solution, and stir until the solid is completely dissolved. Separate layers, discard the dichloromethane layer, add 10 g of activated carbon to...
Embodiment 3
[0060] Synthesis of 2',3'-di-O-acetyl-5'-deoxy-5-fluorocytidine hydroiodide
[0061]
[0062] (1) In a 2L glass reaction bottle, connect the tail gas (ammonia) absorption device for standby, replace with nitrogen for 5 minutes, add 200ml of dichloromethane, add 65g of 5-fluorocytosine and 92ml of hexamethyldisilazide under stirring alkane, 0.2ml iodotrimethylsilane, the temperature of the system was raised to 60-70°C, and the reaction was carried out for 10-15 hours until the feed solution was completely dissolved.
[0063] (2) In another reaction flask, put 300ml of dichloromethane, 130g of 5-deoxy-1,2,3-tri-O-acetyl-β-D-ribofuranose, cool down to 0°C, add trimethyl 110ml of iodosilane, keep warm at 0°C for 3-5 hours.
[0064] (3) Add the protected 5-fluorocytosine reaction solution into the iodide reaction solution, and react at room temperature for 3-5 hours. Then, 50 ml of isopropanol was added to the feed liquid, and a yellow-white solid was precipitated. Then add 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com