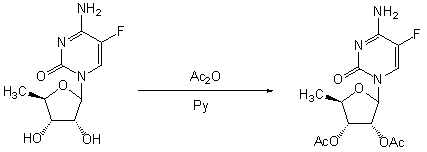
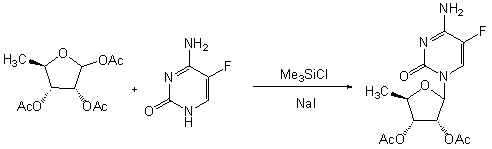
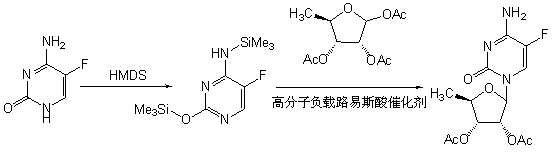
Preparation method of 2',3'-bi-O-acetyl-5'-deoxy-5-fulurocytidine
A technology of acetyl and flucytidine, which is applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the problem of environmental and operator damage, trifluoromethanesulfonic acid is expensive and has no practical value and other problems, to avoid expensive, easy to separate, and shorten the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0034] Example 1 Preparation of 2',3'-di-O-acetyl-5'-deoxy-5-fluorocytidine
[0035] Suspend 5-fluorocytosine (129g, 1mol) in 180mL of anhydrous toluene, add 12mL of anhydrous N,N-dimethylformamide, hexamethyldisilazane (240mL, 1.1mol), and heat for 45min to Completely dissolve at 117°C, cool to 70°C, evaporate to dryness under reduced pressure, add 1,2,3-tri-O-acetyl-5-deoxyribose (234g, 0.9mol) at room temperature, polymer loaded tetrachloride Titanium (50g) was reacted with 1200mL of anhydrous dichloromethane for 3h, filtered, the filter cake was washed with dichloromethane (60mL×3), the filtrate was collected and washed with water (100mL×3), and the organic phase was dried with anhydrous sodium sulfate , filtered, the filtrate was concentrated to dryness under reduced pressure, and the residue was recrystallized with 800 mL of ethanol to obtain 264.7 g of a white solid, with a yield of 89.3%, an optical purity of 99.5%, and a melting point of 189-191°C.
Embodiment example 2
[0036] Example 2 Suspend 5-fluorocytosine (129g, 1mol) in 180mL of anhydrous toluene, add 8mL of anhydrous N , N -Dimethylformamide, hexamethyldisilazane (240mL, 1.1mol), heated to 118°C for 1h to completely dissolve, cooled to 70°C, evaporated to dryness under reduced pressure, added 1,2,3- three- O-Acetyl-5-deoxyribose (312.3g, 1.2mol), polymer loaded ferric chloride (20g) and 1200mL of anhydrous dichloromethane were reacted for 5h, filtered, and the filter cake was dichloromethane (60mL×3) Wash, collect the filtrate and wash with water (100mL×3), dry the organic phase with anhydrous sodium sulfate, filter, concentrate the filtrate to dryness under reduced pressure, and recrystallize the residue with 800mL of ethanol to obtain 268.7g of white solid with a yield of 81.6% , optical purity 99.2%, melting point 189~191°C.
Embodiment example 3
[0037] Example 3 Add 5-fluorocytosine (129g, 1mol) to 180mL anhydrous N , N -In dimethylformamide, add hexamethyldisilazane (240mL, 1.1mol), heat to 119°C for 30min to dissolve completely, cool to 80°C, evaporate to dryness under reduced pressure, add 1,2, 3-three- O -Acetyl-5-deoxyribose (234g, 0.9mol), polymer-loaded copper chloride (150g) and 1500mL of anhydrous 1,2-dichloroethane were reacted for 2h, filtered, and the filter cake was washed with 1,2-bis Wash with ethyl chloride (80mL×3), collect the filtrate and wash with water (100mL×3), dry the organic phase with anhydrous sodium sulfate, filter, concentrate the filtrate to dryness under reduced pressure, and recrystallize the residue with 800mL ethanol to obtain a white solid 258.1g, yield 87.1%, optical purity 98.9%, melting point 188~189℃.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com