Synthesis method of capecitabine
A synthetic method, capecitabine technology, applied in the field of chemical drug synthesis, to achieve the effects of reduced production costs and simplified synthetic processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0027] In order to better understand the present invention, the following will be further described in conjunction with specific examples.
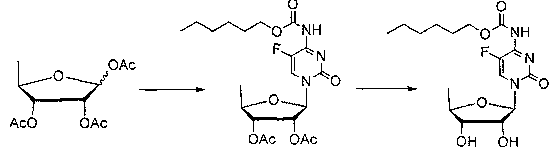
[0028] (1) Put toluene (206 g), HMDS (79.38 g), 5-fluorocytosine (59.58 g), and ammonium sulfate (2.4 g) into a 1000 ml reaction bottle, stir and heat up to reflux, dissolve in about 2 hours, and then reflux For 1 hour, recover the solvent under reduced pressure (about 80°C) to dryness, cool down to below 30°C, add dichloromethane (1010.26g), 5-deoxy-1,2,3-triacetoxy-D-ribose is α, β isomer mixture (120g), in which the molar ratio of α, β isomer is 2.7:1, cool down to 20°C, add trifluoromethanesulfonic acid (8.372ML) dropwise, control < 25°C, drop for about 2 hours After completion, heat preservation reaction at 25°C for 12 hours to obtain reaction solution a;
[0029] (2) Transfer the reaction solution a into a 2000ml reaction bottle, add sodium bicarbonate (232.64g), add water (400g) dropwise, and drop it for about 1-2 hours, stir for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com