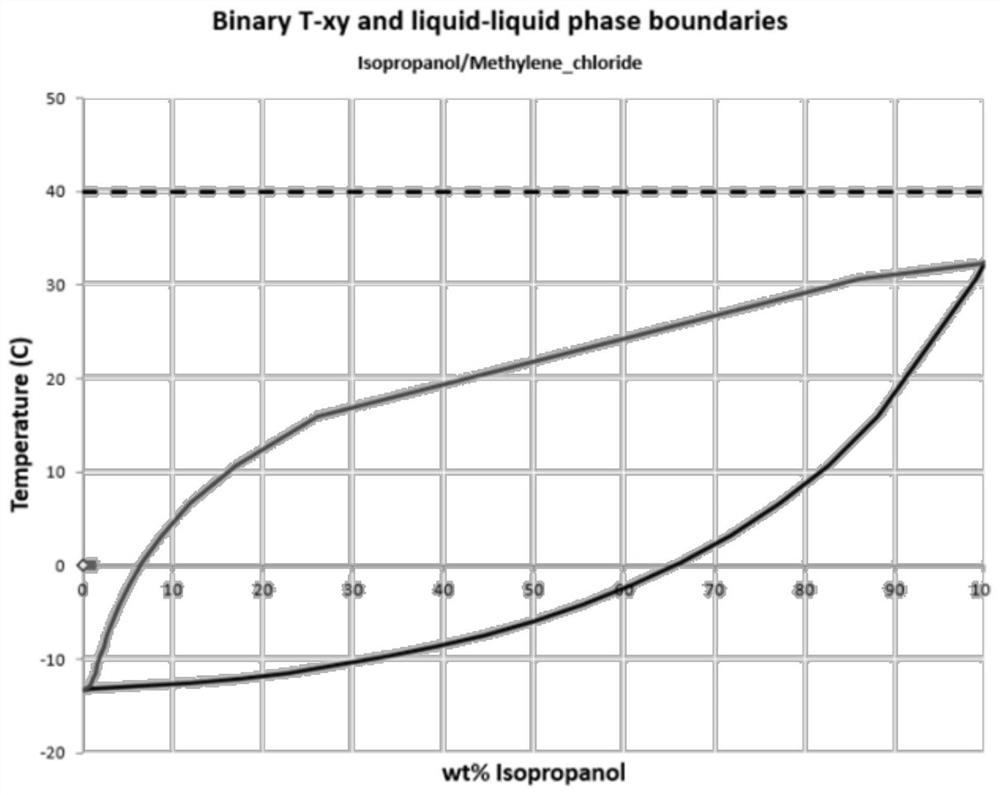
Preparation method of capecitabine intermediate suitable for industrial production
A technology of capecitabine and intermediates, applied in the field of pharmaceutical drug synthesis, can solve the problems of increased industrial production cost, polyisopropanol solvent, and low production efficiency, and achieve stable yield, high product purity, and reduced processing cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0028] A kind of preparation method that is applicable to the capecitabine intermediate of industrialized production, comprises following preparation steps:
[0029] The first step, feeding reaction: After adding acetonitrile, 5-fluorocytosine, hexamethyldisilazane, and trifluoromethanesulfonic acid into the reaction kettle, heat up to reflux, and maintain stirring until the solids are completely dissolved and then continue to reflux At least 2 hours, then lower the temperature to 20~35°C, add 1,2,3-triacetoxy-5-deoxy-D-ribose, then slowly add trifluoromethanesulfonic acid into the reaction kettle through the feeding tank, heat to 45-55°C, and stir for at least 24 hours. In this synthesis temperature range, it is because, in the case of exceeding 55°C, large impurities will be produced, which will affect the yield, and in the case of lower than 45°C, it will affect the reaction. Speed, and finally control the internal temperature of the reactor not higher than 35 ℃.
[0030] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com