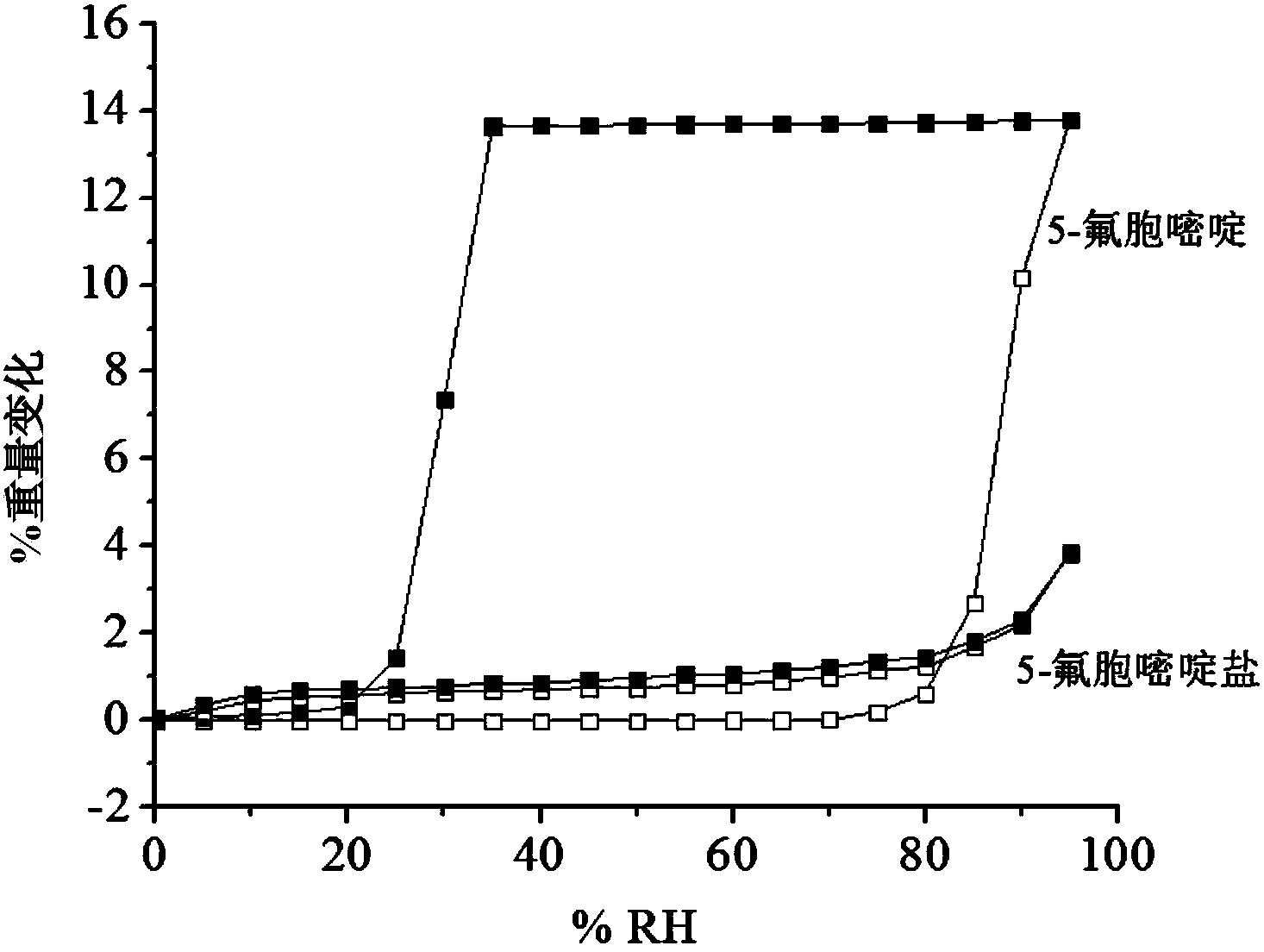
5-flucytosine salt as well as preparation method and application thereof
A technology of flucytosine salt and flucytosine, applied in the field of chemical pharmacy, can solve the problems of reducing the absorption capacity of the small intestine, causing stones, loss of appetite, etc., and achieving the effects of wide boundary conditions, high repeatability and mild conditions of crystallization parameters
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] Further, the preparation method may include: dissolving 5-fluorocytosine and acesulfame potassium in a solvent to form a solution, then filtering, and at least choosing any one of the methods of leaving the solution to stand or removing the solvent in the solution After processing the solution, the obtained colorless transparent crystal or white powder is the final product.
[0033] In the preparation method, the molar ratio of 5-fluorocytosine and acesulfame potassium is preferably 1:1-1:8, 1:3-1:8 or 1:1.
[0034] In this preparation method, the solvent is preferably water.
[0035] In a more specific embodiment, the preparation method of 5-fluorocytosine salt of the present invention may comprise the following steps:
[0036] 1) At a certain temperature, 5-fluorocytosine and acesulfame potassium are completely dissolved in the solvent according to a certain molar ratio to form a supersaturated, saturated or unsaturated solution;
[0037] 2) After filtering, let the...
Embodiment 1
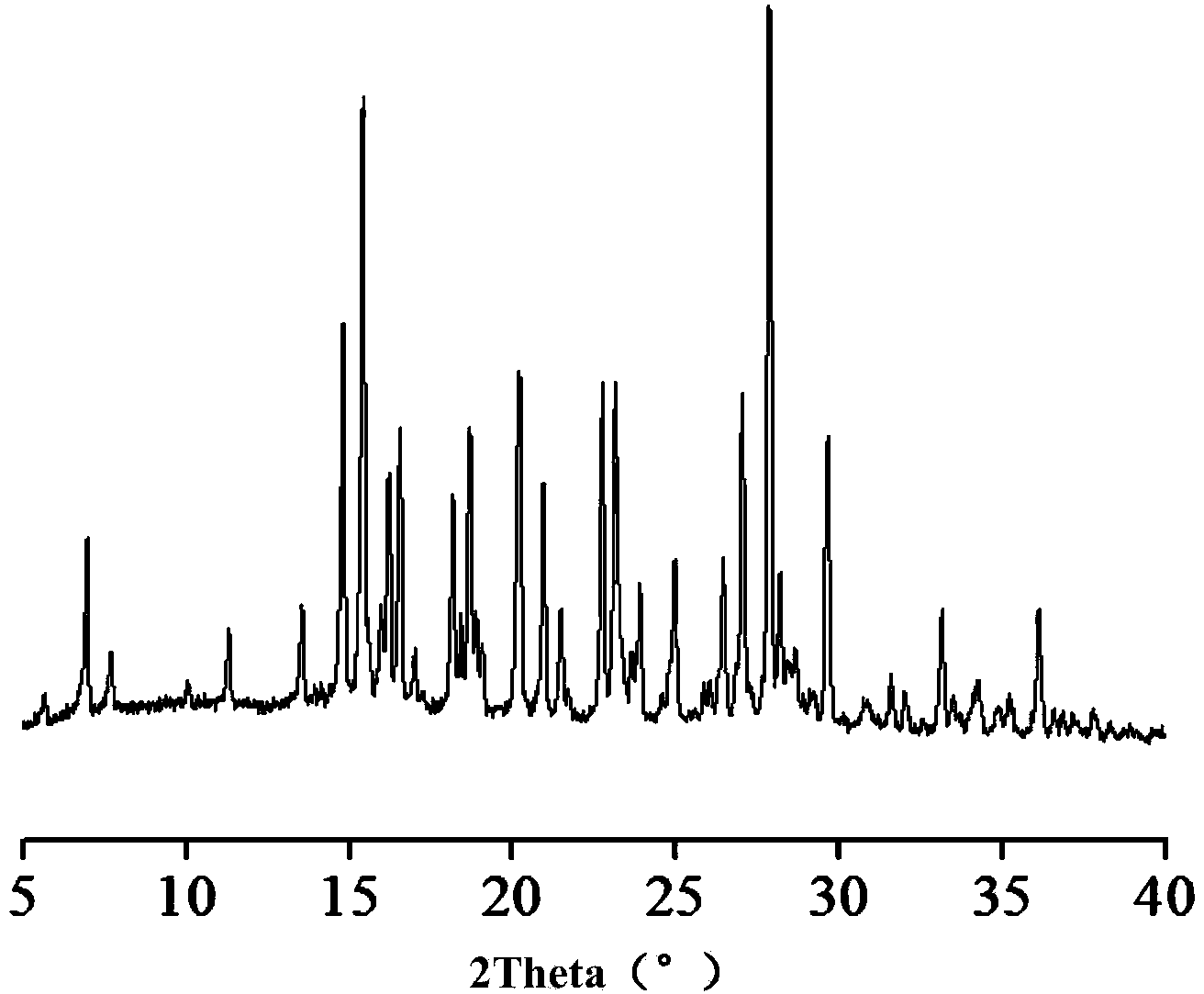
[0051] Example 1: Preparation of salt (that is, 5-fluorocytosine salt, the same below): Weigh 0.2 mmol 5-fluorocytosine and 0.2 mmol acesulfame potassium, dissolve them in 10 mL ultrapure water successively, filter them and put them in liquid nitrogen Freeze and freeze-dry for 72h to obtain 46.8mg of salt. Yield 80.1%. XRD patterns such as figure 2 shown.
Embodiment 2
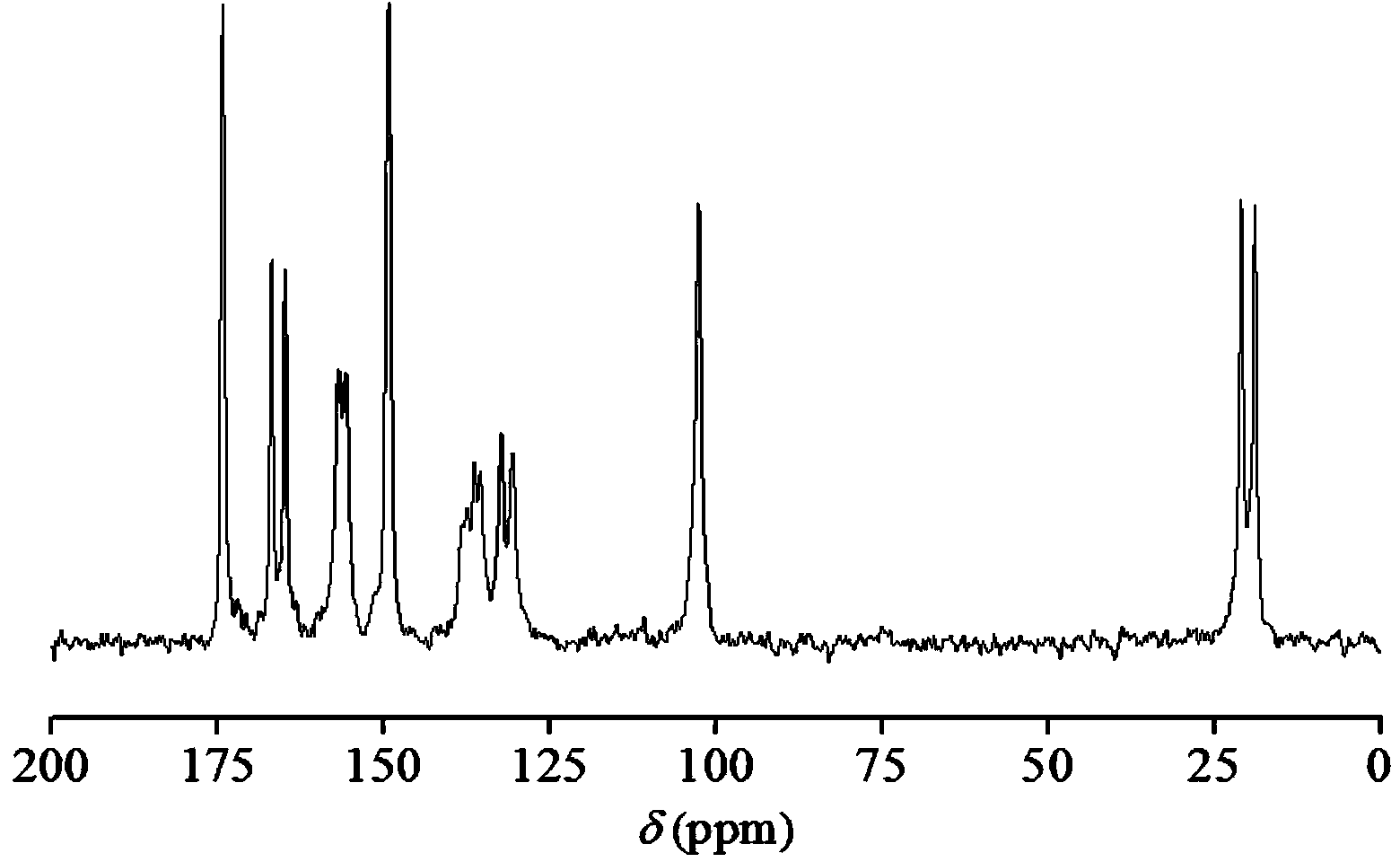
[0052] Example 2: Salt preparation: Weigh 0.4 mmol 5-fluorocytosine and 0.4 mmol acesulfame K, dissolve them in 20 mL ultrapure water successively, filter them, freeze them in liquid nitrogen, freeze-dry for 72 hours, and obtain 94.0 mg of salt. Yield 80.5%. Its solid-state NMR fingerprint image 3 , as shown in 4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com