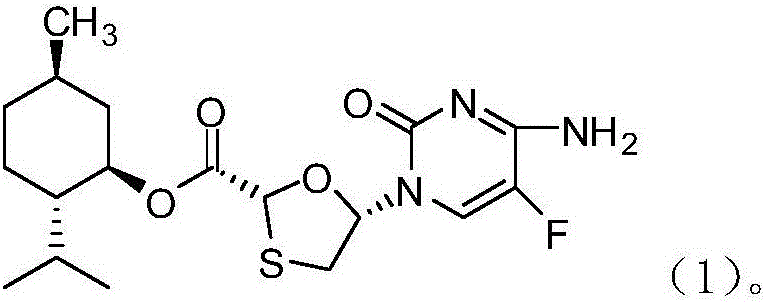
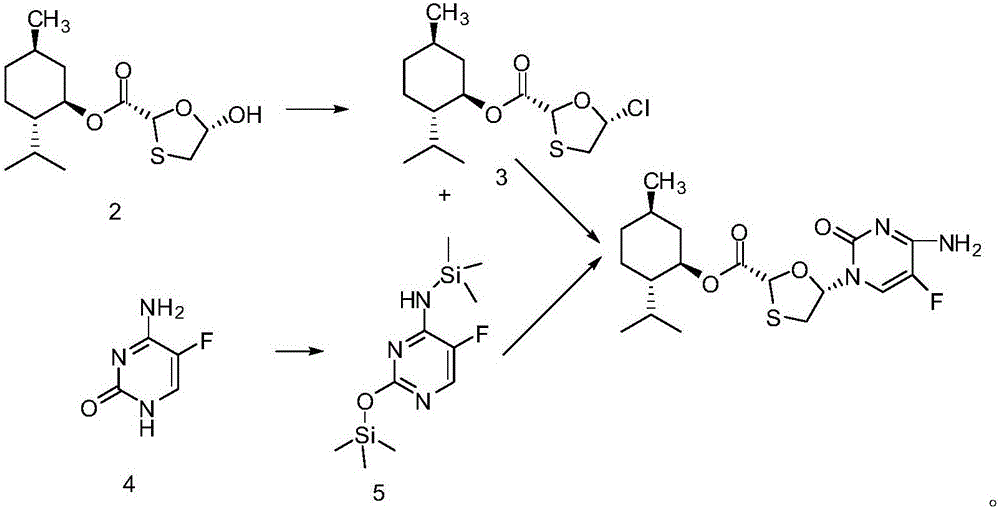
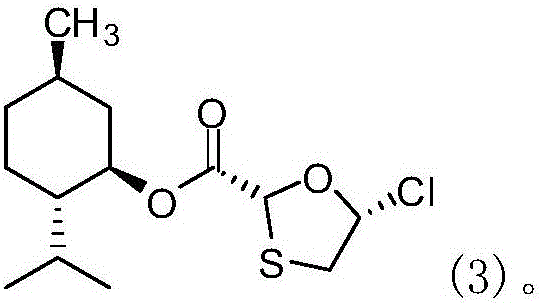
Novel process for preparing emtricitabine intermediate
A technology of emtricitabine and intermediates, which is applied in the field of drug synthesis, can solve the problems of high corrosiveness of reagents, low silanization yield, and low purity of compounds, and achieve the effects of low corrosiveness, high purity, and extended service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053]
[0054] Add DMF (33.5kg, 444.5mol) in reactor, methylene dichloride 725kg, N 2The temperature was lowered to 0°C under protection, and a solution of triphosgene (46kg, 155mol) in dichloromethane (325kg) was added dropwise within about 2.5 hours. After the addition was completed, (2R,5R)-5-hydroxyl-1 was added in batches within 1 hour , 3-Oxathiolane-2-carboxyl-menthyl ester (2) (125kg, 433.5mol), after the addition was completed, it was stirred at 18°C to 20°C for 1 hour, and the feed liquid was a light yellow solution.
Embodiment 2
[0056]
[0057] Add 56.25kg (403.5mol) of 5-fluorocytosine to the reactor, 30.3kg (201.7mol) of sodium iodide, ammonium sulfate (0.75kg, 5.65mol), hexamethyldisiloxane (75kg, 464.7mol) , 300kg of dichloromethane, the suspension was heated to reflux, and after 6 hours, a light yellow clear liquid was obtained.
Embodiment 3
[0058] The preparation technology of the compound described in embodiment 3 formula (1)
[0059]
[0060] 1. Add DMF (33.5kg, 444.5mol), dichloromethane 725kg, N 2 The temperature was lowered to 0°C under protection, and a solution of triphosgene (46kg, 155mol) in dichloromethane (325kg) was added dropwise within about 2.5 hours. After the addition was completed, (2R,5R)-5-hydroxyl-1 was added in batches within 1 hour , 3-oxathiolane-2-carboxyl-menthyl ester (2) (125kg, 433.5mol), after the addition was completed, it was kept stirring at 18°C to 20°C for 1 hour, and the feed liquid was a light yellow solution.
[0061] 2. Add 56.25kg (403.5mol) of 5-fluorocytosine, 30.3kg (201.7mol) of sodium iodide, ammonium sulfate (0.75kg, 5.65mol), hexamethyldisiloxane (75kg , 464.7mol), dichloromethane 300kg, the suspension was heated to reflux, and after 6 hours, a pale yellow clear liquid;
[0062] 3. Cool down to 25°C, add the second-step feed solution below 30°C, a lot of white...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com