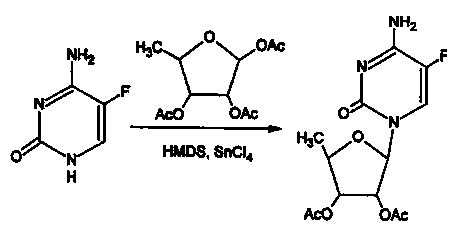
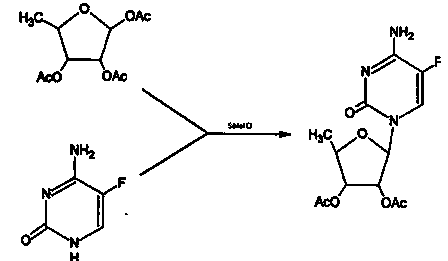
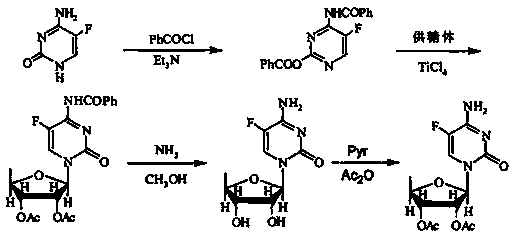
Method for preparing capecitabine intermediate 2', 3'-di-O-acetyl-5'-deoxy-5-fluorocytidine by enzymatic composite chemical method
A capecitabine and chemical method technology, applied in the field of medicinal chemistry, can solve the problems of complicated and complicated preparation method of -deoxy-5-fluorocytidine, unsuitable for production conditions, unsuitable for environmental protection requirements, etc. The effect of high purity, easy control and few by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 5mM 5 ’ -Deoxyribose-1-phosphate was reacted with 5mM 5-fluorocytosine, 25 units / mL pyrimidine nucleoside phosphorylase, 5mM CaCl2, at 28°C for 12 hours. After the end of the reaction, a white precipitate was visible at the bottom of the reactor. The white precipitate was filtered off, and the filtrate was concentrated to dryness at 55°C under reduced pressure. The obtained residue was dissolved in 5ml of methanol, dried by adding 2g of anhydrous sodium sulfate, filtered, and 5ml of isopropyl ether was added dropwise to the filtrate while stirring, and filtered after 2h. The solid was mixed with 40 ℃ vacuum drying for 4 hours, that is, 4.8mM of 5 ’ - Deoxy-5-fluoro-cytidine (yield 96%, HPLC 99.4%). mp192-193°C (literature: 192-194°C). HNMR(DMSO-d6)δ: 1.276 (d, 3H, H-11), 3.644 (m, 1H, H-3), 3.809 (m, 1H, H-4), 3.992 (m, 1H, H-2 ), 4.980 (d, 1H, H-10), 5.277 (d, 1H, H-9), 5.677 (s, 1H, H-1) 7.559 (s, J=7.0Hz, 1H, H-8), 7.753 (m, 2H, H-12).
[0021]
Embodiment 2
[0023] Add 5mM 5 ’ -Deoxyribose-1-phosphate was reacted with 5mM 5-fluorocytosine, 25 units / mL pyrimidine nucleoside phosphorylase, 5mM CaCl2, at 28°C for 12 hours. After the end of the reaction, a white precipitate was visible at the bottom of the reactor. The white precipitate was filtered off, and the filtrate was concentrated to dryness at 55°C under reduced pressure. The obtained residue was dissolved in 5ml of methanol, dried by adding 2g of anhydrous sodium sulfate, filtered, and 5ml of isopropyl ether was added dropwise to the filtrate while stirring, and filtered after 2h. The solid was mixed with 40 ℃ vacuum drying for 4 hours, that is, 4.6mM of 5 ’ -Deoxy-5-fluoro-cytidine (yield 92%, HPLC 99.2%).
Embodiment 3
[0025] Add 5mM 5 ’ -Deoxyribose-1-phosphate was reacted with 5mM 5-fluorocytosine, 25 units / mL pyrimidine nucleoside phosphorylase, 5mM CaCl2, at 28°C for 12 hours. After the end of the reaction, a white precipitate was visible at the bottom of the reactor. The white precipitate was filtered off, and the filtrate was concentrated to dryness at 55°C under reduced pressure. The obtained residue was dissolved in 5ml of methanol, dried by adding 2g of anhydrous sodium sulfate, filtered, and 5ml of isopropyl ether was added dropwise to the filtrate while stirring, and filtered after 2h. The solid was mixed with 40 ℃ vacuum drying for 4 hours, that is, 4.7mM of 5 ’ -Deoxy-5-fluoro-cytidine (yield 94%, HPLC 99.5%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com