Method for preparing capecitabine and hydroxyl derivative intermediate thereof
A technology of hydroxy derivatives and capecitabine, which is applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problems of uneconomical total cost, poor process controllability, and high toxicity, and achieve Avoid the use of toxic or expensive raw materials, good process controllability, and easy industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
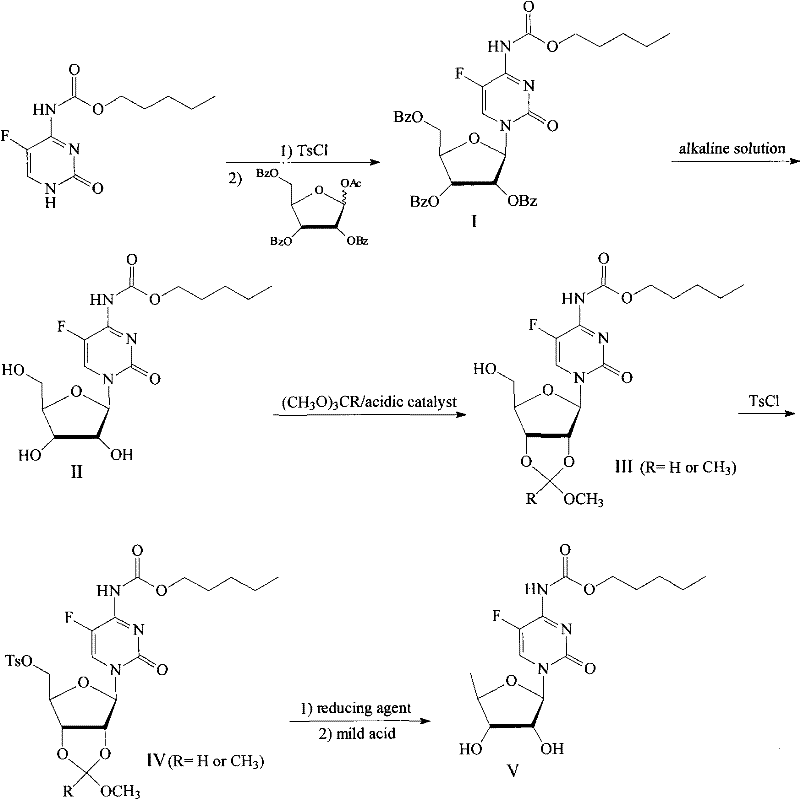
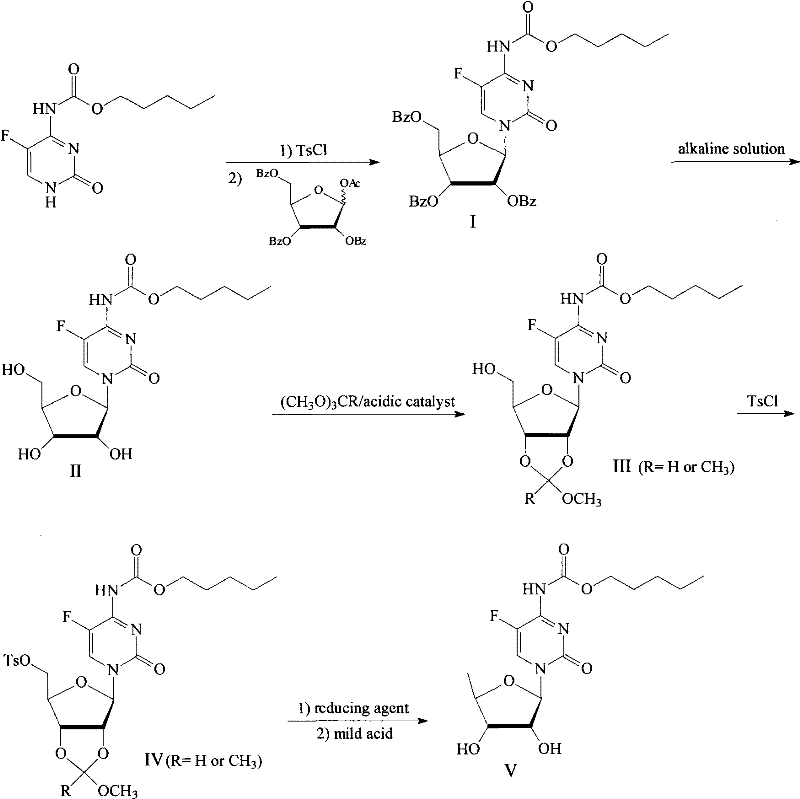
[0020] Example 1: Preparation of 2′, 3′, 5′-tri-O-benzoyl-N-[(n-pentyloxy)carbonyl]-5-fluorocytidine nucleoside (I)
[0021] 25.00g (0.10mol) of N-[(n-pentyloxy)carbonyl]-5-fluorocytosine was dissolved in 1,2-dichloroethane (150mL), and benzoyl chloride (12.52mL, 0.11mol ), stirred and heated to reflux for 1h, slowly added triethylamine (14.0mL, 0.11mol) dropwise, continued to reflux and stir for 5h, cooled to room temperature and filtered, the filter cake was washed with a small amount of 1,2-dichloroethane, and the filtrate and washings were combined , to obtain light brown transparent liquid. Dissolve 55.0g (0.11mol) of 1-O-acetyl-2,3,5-tri-O-benzoyl-D-ribose in the above solution, and add titanium tetrachloride (4.0ml, 0.10 mol) solution was stirred overnight at room temperature. The reaction mixture was poured into ice water (200ml), chloroform (10ml) was added, and the layers were allowed to stand. The organic phase was washed with water, dried over anhydrous magnesium...
Embodiment 2
[0022] Example 2: Preparation of N-[(n-pentyloxy)carbonyl]-5-fluorocytosine nucleoside (II)
[0023] 35.73g (51.96mmol) of 2′, 3′, 5′-tri-O-benzoyl-N-[(n-pentyloxy)carbonyl]-5-fluorocytosine nucleoside was dissolved in 300mL of methanol and added dropwise 320mL (0.5M) methanol solution of sodium methoxide, stirred at room temperature for 24h. Neutralize with 1.0mol / L hydrogen chloride methanol solution to about pH 6.5, filter to remove sodium chloride, and concentrate to dryness under reduced pressure. Dissolve in 20 mL of methanol, filter, and evaporate to dryness. The residue was dissolved in 100 mL of water, benzoic acid was extracted with chloroform, and the aqueous phase was evaporated to dryness under reduced pressure to obtain 18.53 g of white solid N-[(n-pentyloxy)carbonyl]-5-fluorocytosine nucleoside with a yield of 95%. processed for the next reaction.
Embodiment 3
[0024] Example 3: Preparation of 2', 3'-O-(methoxy)methine-N-[(n-pentyloxy)carbonyl]-5-fluorocytosine nucleoside (III)
[0025] Under nitrogen atmosphere, dissolve 13.54g (36.08mmol) N-[(n-pentyloxy)carbonyl]-5-fluorocytidine nucleoside and 75mg (0.40mmol) p-toluenesulfonic acid in 100ml acetocyanide, stir After uniformity, add 5.74g (54.12mmol) trimethyl orthoformate, react at room temperature for 4 hours, evaporate the volatile reagents under reduced pressure, dissolve the crude product in ethyl acetate, wash with 10% aqueous sodium carbonate solution and saturated brine , dried over magnesium sulfate, and the solvent was distilled off under reduced pressure to obtain 13.40 g of white solid 2', 3'-O-(methoxy)methine-N-[(n-pentyloxy)carbonyl]-5-fluorocytosine nucleus Glycosides, the yield was 89%, and were used in the next step without treatment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com