Fluorocytosine tablet and preparation method thereof
The technology of flucytosine tablet and flucytosine agent is applied in the field of medicine to achieve the effects of improving treatment effect, avoiding intestinal bleeding and avoiding double infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
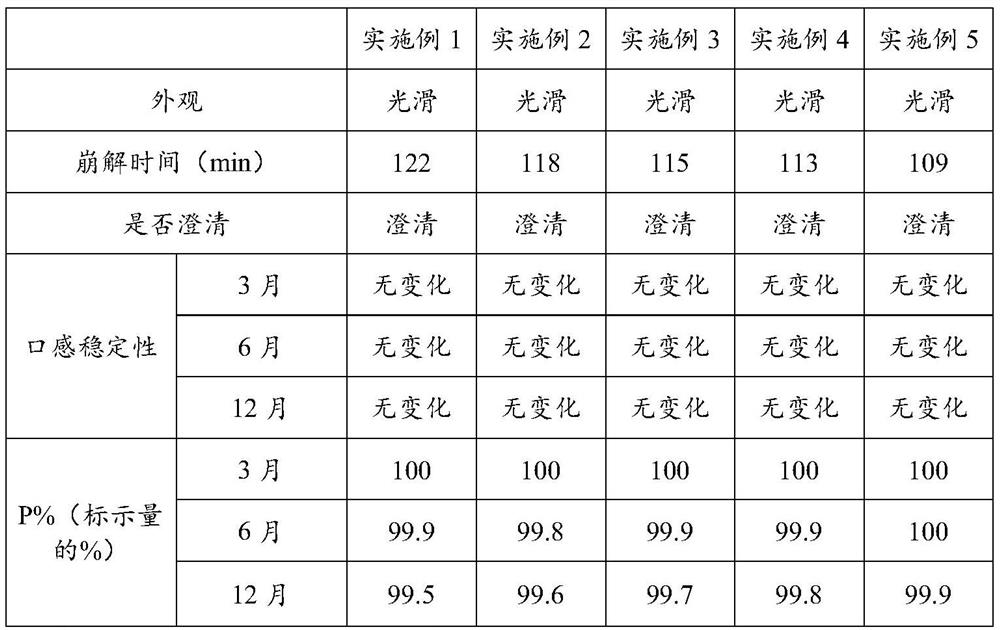
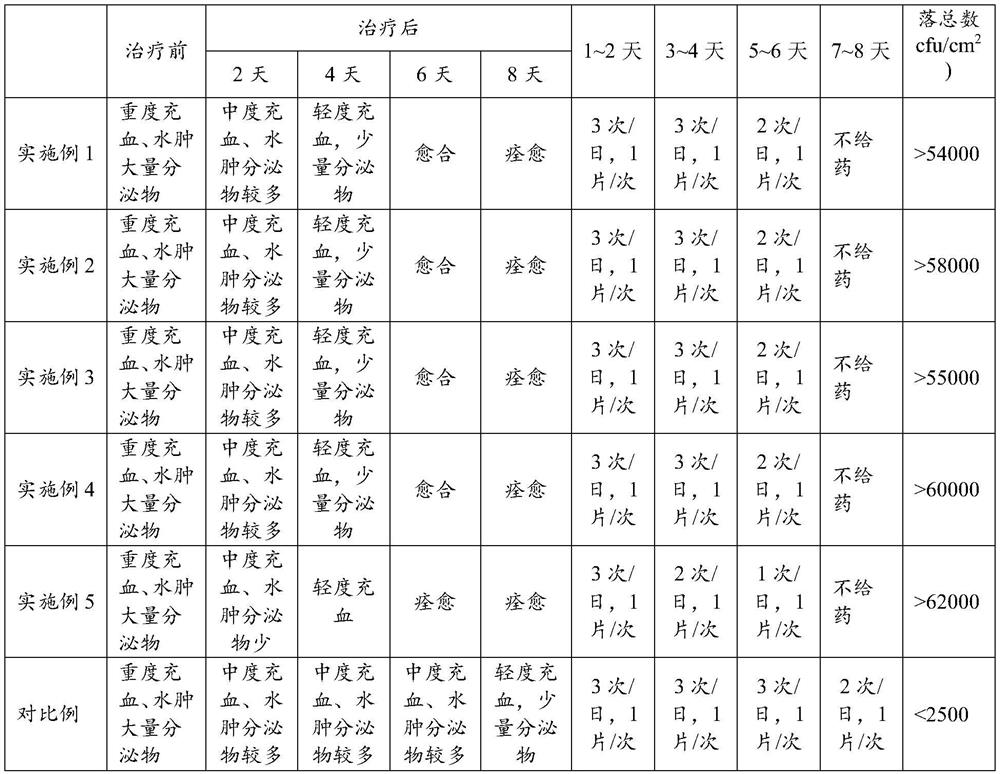
Embodiment 1
[0025] A kind of flucytosine tablet, its preparation method comprises the following steps:
[0026] (1) Pass flucytosine, maltodextrin, sodium carboxymethyl starch, lactulose, and hypromellose through a 80-100 mesh sieve, aztreonam, pea flour, rice bran, brewer's yeast, and compound probiotics The agent is crushed separately and passed through a 200-230 mesh sieve;
[0027] (2) Mix 4.7g of aztreonam, 14g of pea powder, 10g of rice bran, 6g of brewer’s yeast and 3g of compound probiotics, add them to 57g of pure water, stir well and disperse evenly, and pass through a fluidization at a temperature of 50-55°C The bed is dried and crushed at 80-100 mesh to obtain mixture A;
[0028] (3) After mixing 31.2g of flucytosine, 20g of maltodextrin and 3g of lactulose, add it to 163g of pure water, fully stir and disperse evenly, then add 3g of sodium carboxymethyl starch, stir and disperse evenly, Obtain suspension B;
[0029] (4) Spray the mixture A into the suspension B under stirr...
Embodiment 2
[0032] A kind of flucytosine tablet, its preparation method comprises the following steps:
[0033] (1) Pass flucytosine, maltodextrin, sodium carboxymethyl starch, lactulose, and hypromellose through a 80-100 mesh sieve, aztreonam, pea flour, rice bran, brewer's yeast, and compound probiotics The agent is crushed separately and passed through a 200-230 mesh sieve;
[0034] (2) Mix 13.2g of aztreonam, 18g of pea powder, 15g of rice bran, 9g of brewer’s yeast and 6g of compound probiotics, add it to 153g of pure water, stir well and disperse evenly, and pass through a fluidization at a temperature of 50-55°C The bed is dried and crushed at 80-100 mesh to obtain mixture A;
[0035] (3) Mix 67.8g of flucytosine, 30g of maltodextrin and 6g of lactulose and add it to 363g of pure water, fully stir and disperse evenly, then add 1 / 3 of sodium carboxymethyl starch 5g, stir and disperse evenly, Obtain suspension B;
[0036] (4) Spray the mixture A into the suspension B under stirrin...
Embodiment 3
[0039] A kind of flucytosine tablet, its preparation method comprises the following steps:
[0040] (1) Pass flucytosine, maltodextrin, sodium carboxymethyl starch, lactulose, and hypromellose through a 80-100 mesh sieve, aztreonam, pea flour, rice bran, brewer's yeast, and compound probiotics The agent is crushed separately and passed through a 200-230 mesh sieve;
[0041] (2) Mix 7.2g of aztreonam, 15g of pea flour, 12g of rice bran, 7g of brewer’s yeast and 4g of compound probiotics, add them to 82g of pure water, stir well and disperse evenly, and pass through a fluidization at a temperature of 50-55°C The bed is dried and crushed at 80-100 mesh to obtain mixture A;
[0042] (3) After mixing 41.3g of flucytosine, 24g of maltodextrin and 3g of lactulose, add it to 140g of pure water, stir well and disperse evenly, then add 4g of sodium carboxymethyl starch, stir and disperse evenly, Obtain suspension B;
[0043] (4) Spray the mixture A into the suspension B under stirrin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com