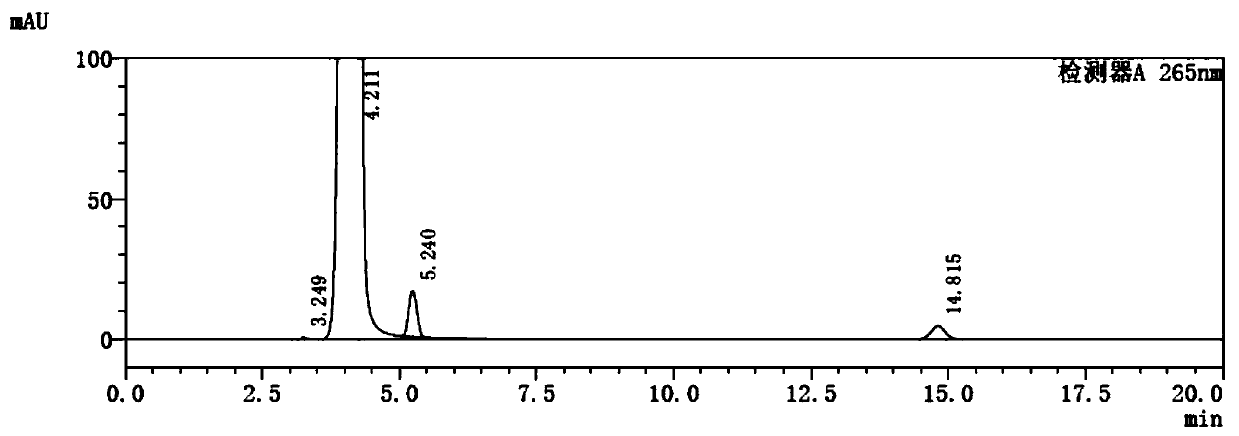
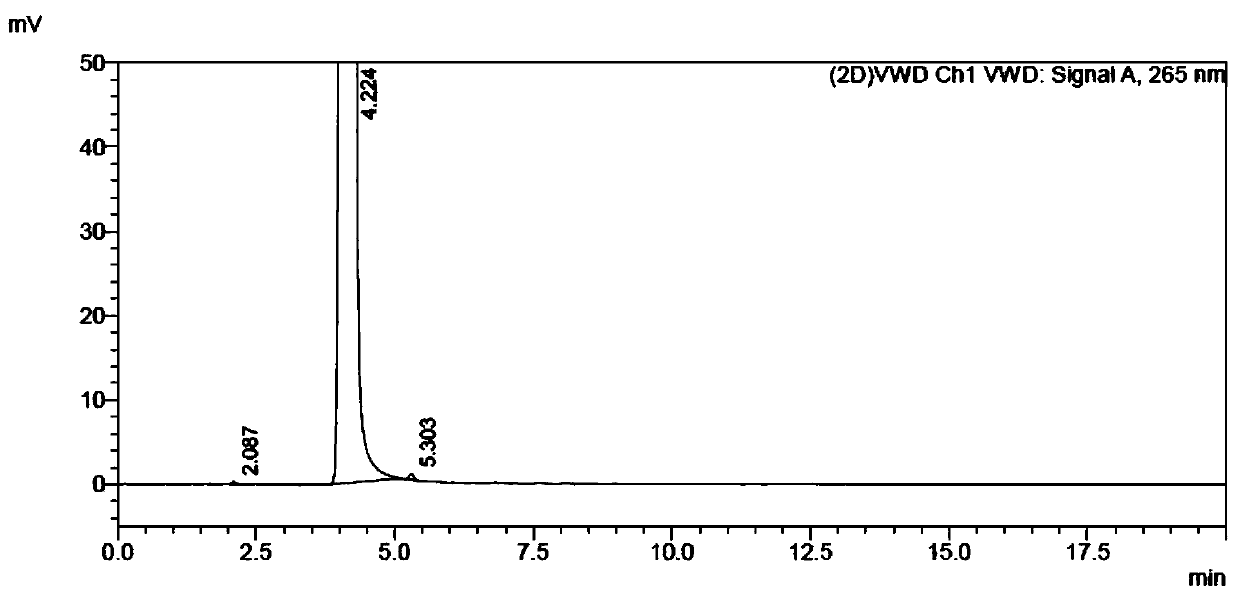
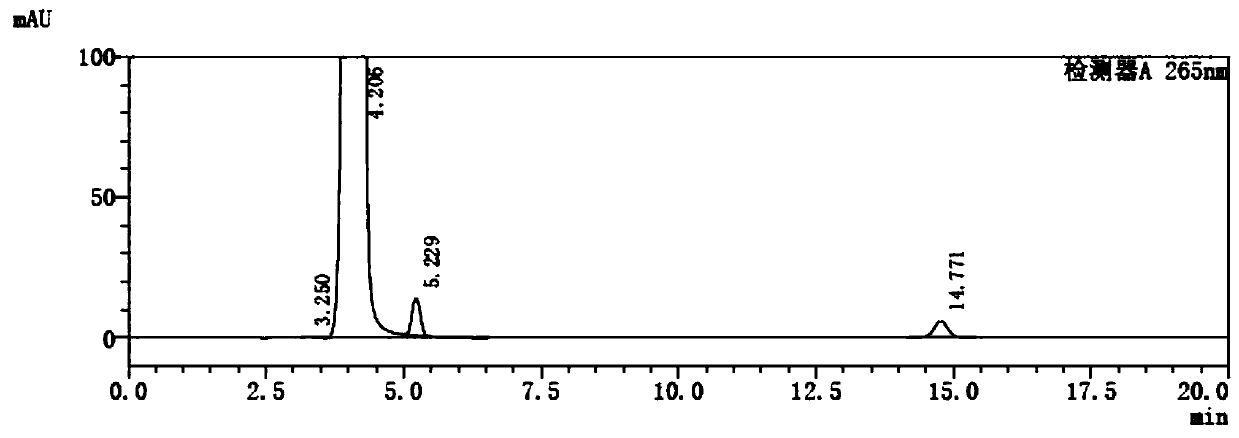
A kind of preparation method of 5-fluorocytosine
A technology of flucytosine and fluoroacetonitrile, which is applied in the field of pharmaceutical chemical synthesis, can solve the problems of increased production cost and low reaction yield, and achieve the effects of prolonging service life, high purity, and improving atom utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] This embodiment provides a preparation method of 5-fluorocytosine, specifically, comprising the following steps:
[0040] Step 1: firstly add an organic solvent, fluoroacetonitrile, ethyl formate and an organic base into the autoclave, heat up the reaction in a carbon monoxide environment, then release the pressure and lower the temperature to obtain the first reaction solution containing the intermediate 1.
[0041] Among them, after passing the pressure of carbon monoxide, the pressure in the autoclave rises to 1.0-1.5MPa. When the temperature is raised in the carbon monoxide environment, the reaction temperature is 90-105°C, the reaction time is 8h-16h, and the pressure in the autoclave is maintained at 3.0MPa- 3.5MPa, when the pressure is released and the temperature is lowered to -5°C ~ -10°C, the mass ratio of fluoroacetonitrile to ethyl formate is 2.5:1 ~ 3.5:1. In this example, the mass ratio of fluoroacetonitrile to ethyl formate It is 2.5:1.
[0042] In this ...
Embodiment 2
[0083] This embodiment provides a method for preparing 5-fluorocytosine, the reaction steps and reaction mechanism are the same as those in Example 1, and the specific operations are as follows:
[0084] Step 1, add 170kg of toluene to the 1000L autoclave, then add 17.5kg of fluoroacetonitrile, 5.0kg of ethyl formate and 15kg of sodium methoxide, replace the air in the autoclave with nitrogen, and then replace the nitrogen in the autoclave with carbon monoxide, Continuously feed carbon monoxide to raise the pressure in the autoclave to 1.5Mpa, raise the temperature to 105°C for reaction under agitation, and keep the pressure in the autoclave at 3.5MPa. After 16 hours of reaction, cool down to below 50°C, release the pressure, and then transfer the reaction solution Cool down to a 1000L autoclave to -10°C to obtain a reaction solution containing Intermediate 1.
[0085] In this embodiment, the mass ratio of fluoroacetonitrile to ethyl formate is 3.5:1.
[0086] Step 2: Add met...
Embodiment 3
[0100] This embodiment provides a method for preparing 5-fluorocytosine, the reaction steps and reaction mechanism are the same as those in Example 1, and the specific operations are as follows:
[0101] Step 1, add 170kg of toluene to the 1000L autoclave, then add 15.3kg of fluoroacetonitrile, 5.0kg of ethyl formate and 15kg of sodium methoxide, replace the air in the autoclave with nitrogen, and then replace the nitrogen in the autoclave with carbon monoxide, Continuously feed carbon monoxide to increase the pressure in the autoclave to 1.3Mpa, raise the temperature to 100°C for reaction under stirring conditions, and keep the pressure in the autoclave at 3.2MPa, after 12 hours of reaction, cool down to below 50°C, release the pressure, and then transfer the reaction solution Cool down to a 1000L autoclave to -8°C to obtain a reaction solution containing Intermediate 1. Same as in Example 1, ethyl formate and fluoroacetonitrile react under the action of sodium methoxide to p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com