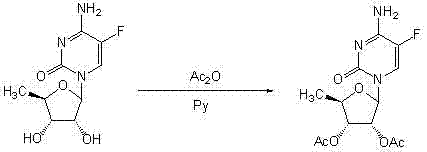
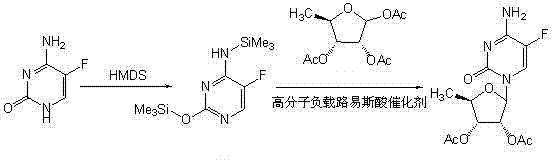
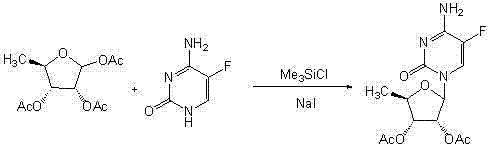A kind of preparation method of 2',3'-di-o-acetyl-5'-deoxy-5-fluorocytidine
A technology of acetyl and flucytidine, which is applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the problem of environmental and operator damage, high cost of trifluoromethanesulfonic acid, and no practical value. and other problems, to avoid expensive, easy separation, and shorten the reaction time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0034] Example 1 Preparation of 2’,3’-Di-O-acetyl-5’-deoxy-5-fluorocytidine
[0035] Suspend 5-fluorocytosine (129g, 1mol) in 180mL anhydrous toluene, add 12mL anhydrous N,N-dimethylformamide, hexamethyldisilazane (240mL, 1.1mol), and heat to Dissolve completely at 117°C, cool to 70°C, evaporate to dryness under reduced pressure, add 1,2,3-tri-O-acetyl-5-deoxyribose (234g, 0.9mol), polymer-supported tetrachloride at room temperature Titanium (50g) and 1200mL of anhydrous dichloromethane were reacted for 3h, filtered, the filter cake was washed with dichloromethane (60mL×3), the filtrate and washing liquid were collected and washed with water (100mL×3), and the organic phase was dried with anhydrous sodium sulfate After filtering, the filtrate was concentrated to dryness under reduced pressure, and the residue was recrystallized with 800 mL of ethanol to obtain 264.7 g of white solid. The yield was 89.3%, the optical purity was 99.5%, and the melting point was 189~191°C.
Embodiment example 2
[0036] Implementation case 2 Suspend 5-fluorocytosine (129g, 1mol) in 180mL anhydrous toluene, add 8mL anhydrous N , N -Dimethylformamide, hexamethyldisilazane (240mL, 1.1mol), heated to 118℃ for 1h, all dissolved, cooled to 70℃, evaporated to dryness under reduced pressure, add 1,2,3- at room temperature three- O -Acetyl-5-deoxyribose (312.3g, 1.2mol), macromolecule-supported ferric chloride (20g) and 1200mL of anhydrous dichloromethane react for 5h, filter, filter cake with dichloromethane (60mL×3) Wash, collect the filtrate and washing liquid (100mL×3), dry the organic phase with anhydrous sodium sulfate, filter, concentrate the filtrate to dryness under reduced pressure, and recrystallize the residue with 800mL ethanol to obtain 268.7g of white solid, the yield is 81.6% , The optical purity is 99.2%, the melting point is 189~191℃.
Embodiment example 3
[0037] Implementation case 3 Add 5-fluorocytosine (129g, 1mol) to 180mL anhydrous N , N -To dimethylformamide, add hexamethyldisilazane (240mL, 1.1mol), heat to 119°C for 30 minutes, and then cool to 80°C, evaporate to dryness under reduced pressure, add 1,2, 3-Three- O -Acetyl-5-deoxyribose (234g, 0.9mol), macromolecule supported copper chloride (150g) and 1500mL of anhydrous 1,2-dichloroethane react for 2h, filter, filter cake with 1,2-di Wash with ethyl chloride (80mL×3), collect the filtrate and wash with water (100mL×3), dry the organic phase with anhydrous sodium sulfate, filter, concentrate the filtrate to dryness under reduced pressure, and recrystallize the residue with 800mL ethanol to obtain a white solid 258.1g, yield 87.1%, optical purity 98.9%, melting point 188~189℃.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com