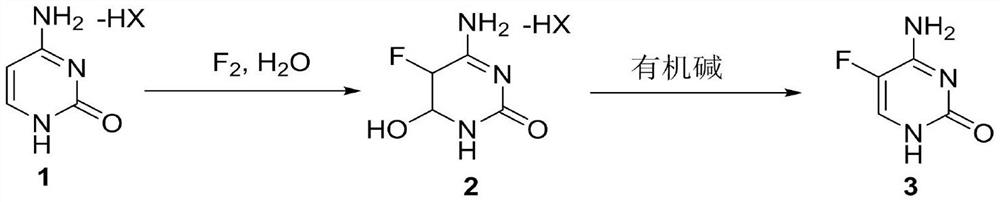
A method for synthesizing 5-fluorocytosine
A technology of fluorocytosine and cytosine, which is applied in the field of synthesizing 5-fluorocytosine, can solve problems such as high cost of formic acid, expensive fluorine reagents, and expensive reaction equipment, and achieve the goal of simplifying production operations, reducing risk factors, and reducing raw material costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
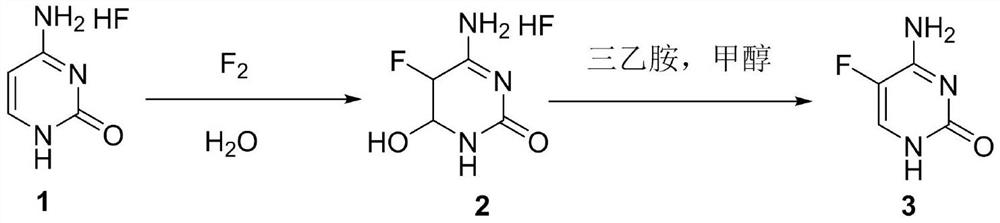
[0028] In the first step, 140g (0.30mol) of cytosine hydrogen fluoride and 400mL of water were added in the reactor, and after stirring evenly, the temperature was raised to 40-45°C, and 12.5g (0.33mol) of 10% fluorine gas was slowly introduced into the liquid phase to track the reaction. When the raw materials basically disappeared, the temperature was lowered to 0° C. to separate out solids, the filter cake was suction filtered, and then rinsed with a small amount of ice water to obtain Intermediate 2, which was dried to obtain 45.1 g (0.27 mol) and put into the next reaction.
[0029] In the second step, 45.1 g (0.27 mol) of intermediate 2 was added in the reaction kettle, then 300 mL of methanol and 41 g (0.41 mol) of triethylamine were added, the temperature was raised to reflux, and then the reaction was incubated for 5 h. After the reaction was completed, the temperature was lowered to 10° C. A large amount of solid was precipitated, and the suction filtratio...
Embodiment 2
[0031]
[0032] In the first step, 140g (0.30mol) of cytosine hydrogen fluoride and 400mL of water were added in the reactor, and after stirring evenly, the temperature was raised to 40-45°C, and 12.5g (0.33mol) of 20% fluorine gas was slowly introduced into the reaction vessel, and the liquid phase tracked the reaction. When the raw materials basically disappeared, the temperature was lowered to 0° C. to separate out a large amount of solid, suction filtration, and the filter cake was rinsed with a small amount of ice water to obtain Intermediate 2, which was dried to obtain 47 g (0.28 mol).
[0033] In the second step, in the reactor, add 47g (0.28mol) of Intermediate 2, then add 300mL of methanol and 42.4g (0.42mol) of triethylamine, heat up to reflux, then keep the temperature for 5h, and cool down to 10°C after the reaction is complete A large amount of solid was precipitated, suction filtration, and the filter cake was rinsed with a small amount of methanol to obtain c...
Embodiment 3
[0035]
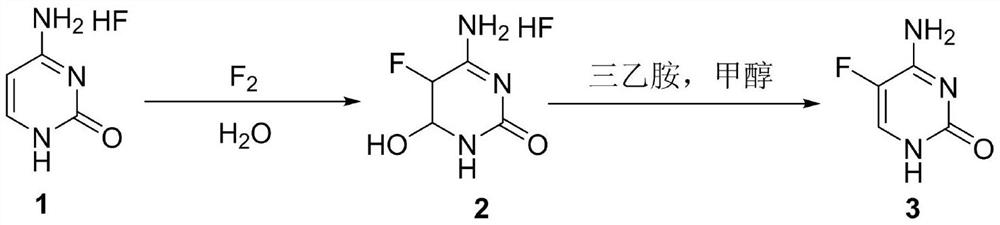
[0036] In the first step, 44.1g (0.30mol) of cytosine hydrochloride and 400mL of water were added to the reactor, and after stirring, the temperature was raised to 40-45°C, and 12.5g (0.33mol) of 20% fluorine gas was slowly introduced into the reactor, and the liquid phase tracked. The reaction was carried out until the raw materials basically disappeared, and the temperature was lowered to 0° C. to precipitate a large amount of solids. The filter cake was suction filtered and washed with a small amount of ice water to obtain Intermediate 2, which was dried to obtain 40 g (0.22 mol).
[0037] In the second step, in the reactor, add 40g (0.22mol) of intermediate 2, then add 300mL of methanol and 33.3g (0.33mol) of triethylamine to heat up and reflux for 5h, heat up to reflux, then heat for 5h, and the reaction ends After cooling to about 2 ℃, a large amount of solids precipitated, and the filter cake was suction filtered and washed with a small amount of methanol to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com