Umbilical cord mesenchymal stem cell preserving fluid
A technology of high-quality stem cells and preservation solution, applied in the field of stem cells, can solve the problems of not being able to inject directly into the human body, long time, large cell damage, etc., and achieve the effect of improving cell survival rate, facilitating subsequent use, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
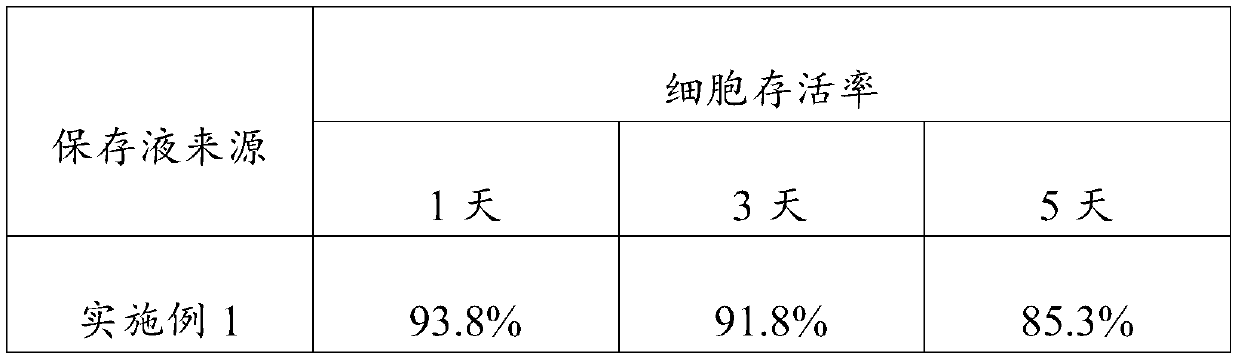
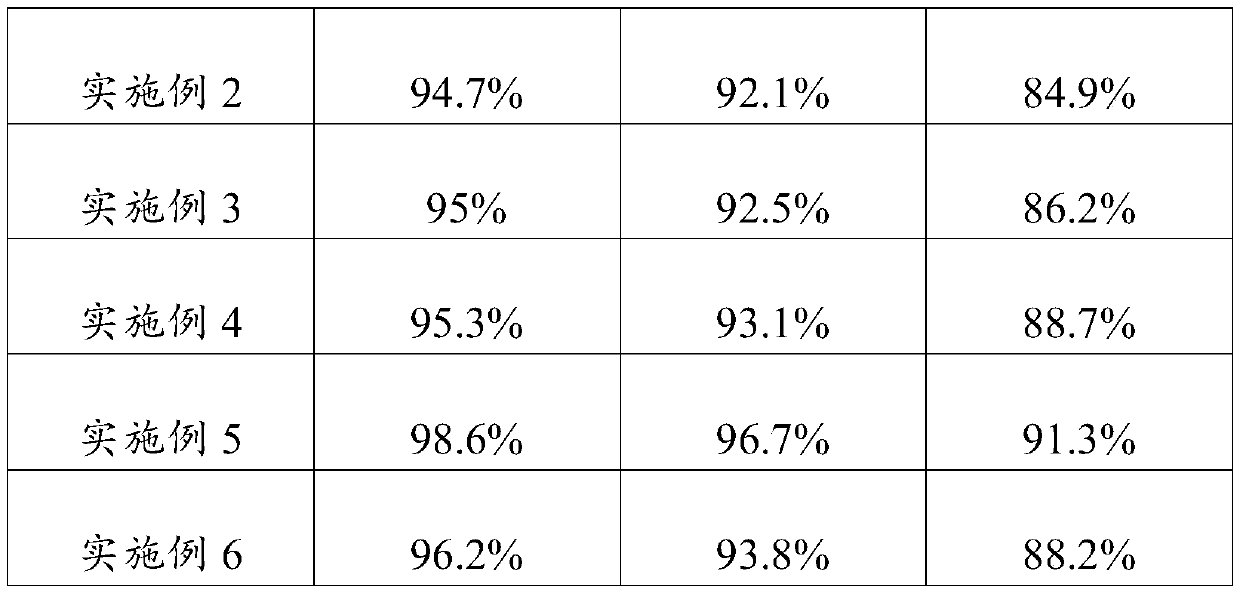
Embodiment 1
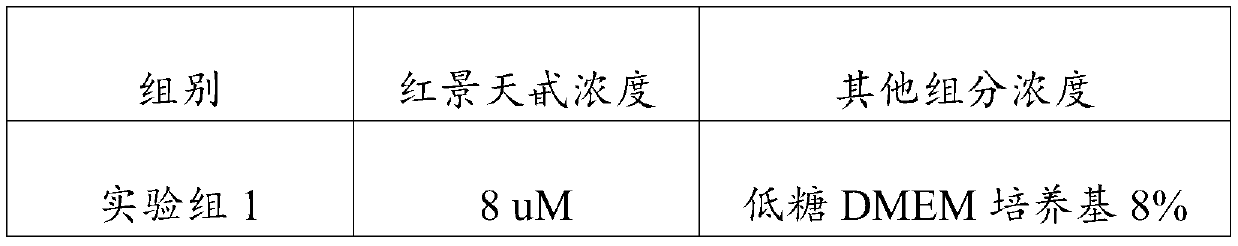
[0023] An embodiment of the umbilical cord mesenchymal stem cell preservation solution of the present invention, the umbilical cord mesenchymal stem cell preservation solution comprises components of the following concentrations: low-sugar DMEM medium 5% (V / V), human serum albumin 18g / L , sodium chloride 10g / L, low molecular weight heparin sodium 2500AXaIU / mL, glucose 40g / L, vitamin C100mg / L and salidroside 10uM.
Embodiment 2
[0025] An embodiment of the umbilical cord mesenchymal stem cell preservation solution of the present invention, the umbilical cord mesenchymal stem cell preservation solution comprises components of the following concentrations: low-sugar DMEM medium 8% (V / V), human serum albumin 15g / L , sodium chloride 9g / L, low molecular weight heparin sodium 3000AXaIU / mL, glucose 25g / L, vitamin C 120mg / L and salidroside 28uM.
Embodiment 3
[0027] An embodiment of the umbilical cord mesenchymal stem cell preservation solution of the present invention, the umbilical cord mesenchymal stem cell preservation solution comprises components of the following concentrations: low-sugar DMEM medium 9% (V / V), human serum albumin 20g / L , sodium chloride 8g / L, low molecular weight heparin sodium 2800AXaIU / mL, glucose 50g / L, vitamin C 150mg / L and salidroside 30uM.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com