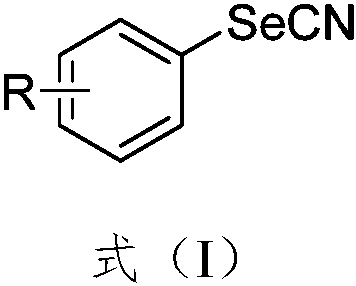
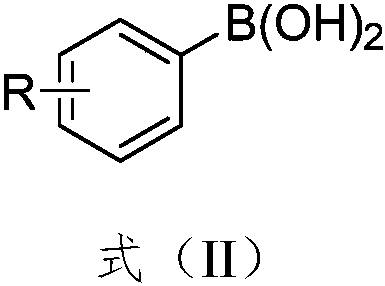
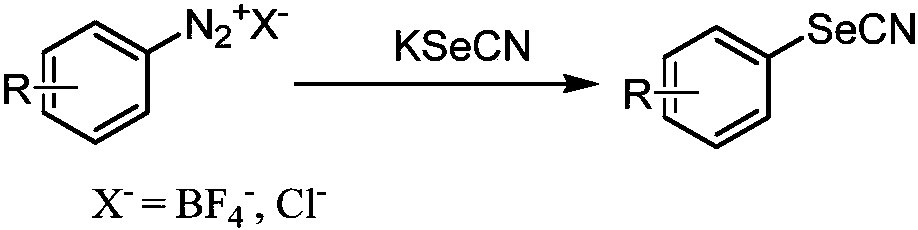
Synthesis method of aryl selenocyanate compounds
A synthesis method and aryl selenium technology, applied in organic chemistry and other directions, can solve problems such as limiting reaction expansion, and achieve the effects of good tolerance, high yield and low environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1. Synthesis of 2-methylbenzeneselenocyanine.
[0048] Add 27.2 mg of o-tolueneboronic acid, 115.3 mg of potassium selenocyanate, 30.2 mg of tetrabutylammonium acetate and 10 mL of acetonitrile into a three-port reaction tube equipped with a magnetic stirring bar. Under the conditions of air and room temperature, the reaction was carried out by electricity for 3 hours, and the constant current was 10mA. After the reaction was completed, it was simply filtered, concentrated by rotary evaporation, and put on the column. Using pure petroleum ether and 2% ethyl acetate as mobile phases in turn, 31.4 mg of 2-methylbenzeneselenocyanate was obtained with a yield of 80%.
[0049] Product 2-methylphenylselencyanide: 1 H NMR (400MHz, CDCl 3 ): δ7.72(d, J=7.6Hz, 1H), 7.22(t, J=7.6Hz, 1H), 2.49(s, 3H); 13 C NMR (100MHz, CDCl 3 ): δ139.9, 134.0, 131.2, 130.3, 127.8, 123.0, 101.2, 22.3.
Embodiment 2
[0050] Example 2. Synthesis of 4-methylphenylselencyanide.
[0051] Add 27.2 mg of p-tolueneboronic acid, 115.3 mg of potassium selenocyanate and 10 mL of acetonitrile into a three-port reaction tube equipped with a magnetic stirring bar. Under the conditions of air and room temperature, electricity was reacted for 5 hours, and the constant current was 10mA. After the reaction was completed, it was simply filtered, concentrated by rotary evaporation, and put on the column. Using pure petroleum ether and 2% ethyl acetate as mobile phases in turn, 25.9 mg of 4-methylphenylselenocyanate was obtained with a yield of 66%.
[0052] Product 4-methylphenylselencyanide: 1 H NMR (400MHz, CDCl 3): δ7.53(d, J=8.0Hz, 2H), 7.21(d, J=8.0Hz), 2.38(s, 3H); 13 C NMR (100MHz, CDCl 3 ): δ140.4, 133.3, 131.1, 117.9, 101.7, 21.2.
Embodiment 3
[0053] Example 3. Synthesis of 2,6-dimethylbenzenselencyanide.
[0054] Add 30.0 mg of 2,6-dimethylphenylboronic acid, 115.3 mg of potassium selenocyanate, 8.2 mg of sodium acetate and 10 mL of acetonitrile into a three-port reaction tube equipped with a magnetic stirring bar. Under the conditions of air and room temperature, electricity was reacted for 5 hours, and the constant current was 15mA. After the reaction was completed, it was simply filtered, concentrated by rotary evaporation and put on the column. Using pure petroleum ether and 2% ethyl acetate as the mobile phase in turn, 17.7 mg of 2,6-dimethylbenzenselencyanide was obtained with a yield of 42%.
[0055] Product 2,6-dimethylbenzenselencyanide: 1 H NMR (400MHz, CDCl 3 ): δ7.23(t, J=7.6Hz, 1H), 7.16(d, J=7.6Hz, 2H); 13 C NMR (100MHz, CDCl 3 ): δ143.1, 130.9, 128.8, 123.9, 101.1, 24.5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com