Methods of producing bioengineered neuronal organoids (BENOS) and uses thereof
A bioengineering and neuron technology, applied in biochemical equipment and methods, microorganisms, biological tests, etc., can solve the problem of lack of neuron network function in organoids, limit disease modeling and drug development, limit neuron function and plasticity, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0233] Embodiment 1: Optimizing the scheme of producing BENO
[0234] To optimize the disclosed protocol for neural induction, NPC amount and neuronal differentiation, the following studies were performed. The data obtained allowed to establish an optimal protocol for the production of BENO (Example 2).
[0235] neural induction optimization
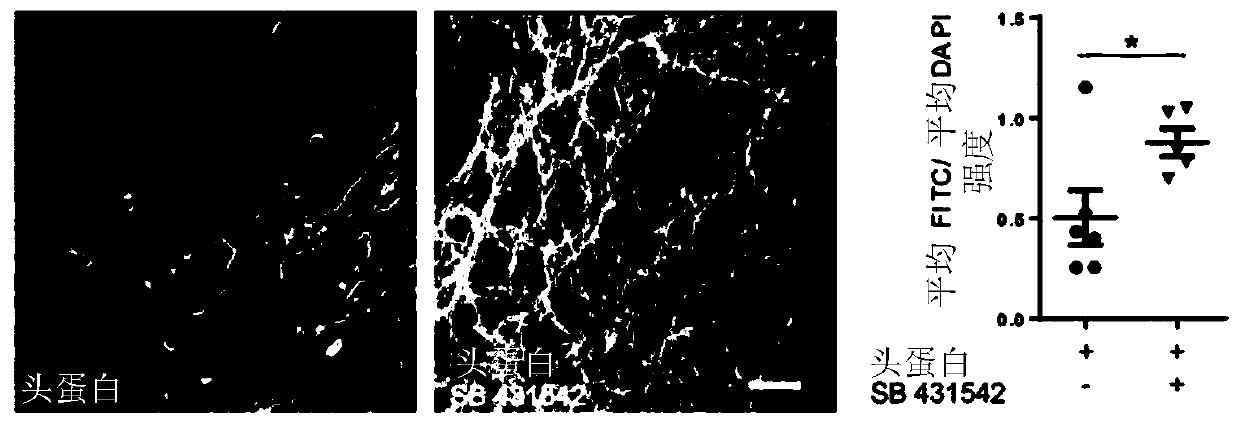
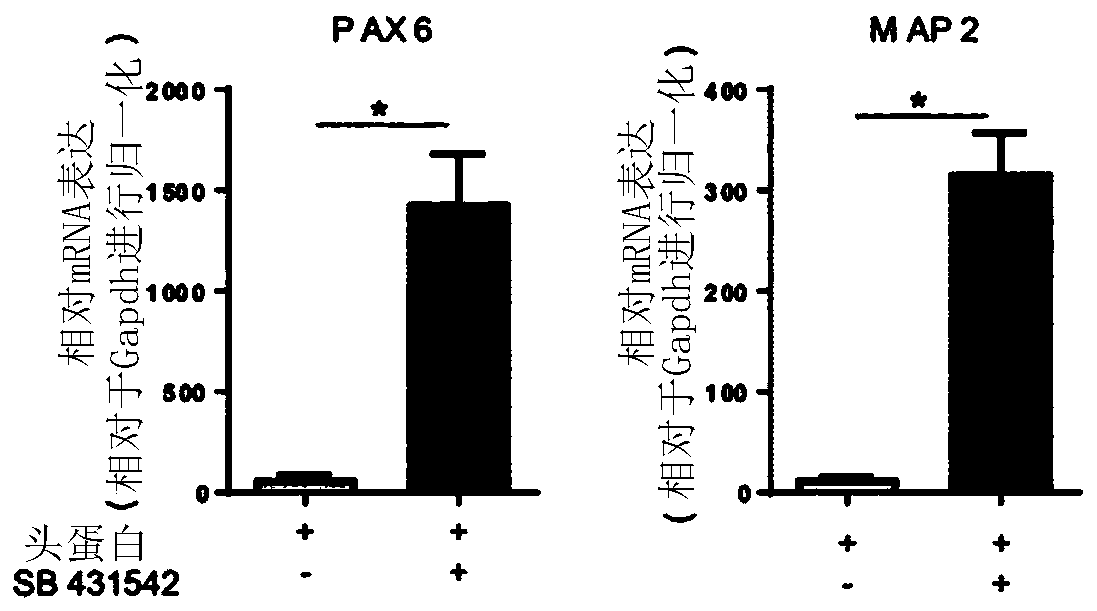
[0236] In initial attempts, a single SMAD pathway inhibition (using Noggin) was used to elicit neural induction, with the addition of retinoic acid (RA). In the present study, it was tested whether dual SMAD signaling pathway inhibition using Noggin and SB431542 during the neural induction phase (days 0–8) might further enhance neurogenesis, for example at day 28. Therefore, NCMs containing only Noggin or NCMs containing Noggin and SB431542 were investigated, respectively. As analyzed by immunofluorescence ( Figure 2A ) and transcript analysis of PAX6 and MAP2 ( Figure 2B ), it was clearly observed that dual SMAD inhibition is ben...
Embodiment 2
[0248] Example 2: Optimization Protocol for Generation of Human Bioengineered Neuronal Organoids (BENO)
[0249] The following studies established optimal protocols for producing human bioengineered neuronal organoids (BENOs).
[0250] Material
[0251] The materials used in these experiments were the same as those used in Example 1.
[0252] Stock solution (-20℃)
[0253] The stock solutions used in these experiments were the same as those used in Example 1.
[0254] Working solution (4°C)
[0255] EDTA 0.5mM solution, 1:30 Matrigel solution, TeSR TM -E8 TM The medium and base medium are the same as those used in Example 1.
[0256] neural presence medium (NCM)
[0257] Basal medium (50 ml) was supplemented with 10 μM SB 431542 (50 μl), 50 ng / ml Noggin (10 μl) and 1 μM RA (5 μl).
[0258] neural progenitor cell expansion medium (NPEM)
[0259] Basal medium (50 ml) supplemented with 10 ng / ml FGF-2 (50 μl) and 5 ng / ml TGFB1.
[0260] neural differentiation...
Embodiment 3
[0277]Example 3: High-throughput transcriptome analysis of BENO
[0278] To characterize the cell types that emerged at different time points (e.g., days -1, 0, 3, 8, 15, 28, 40, 50, and 60) during BENO tissue production, cells were subjected to RNAseq analysis; each time point 3-6 tissues).
[0279] First, the differentiation status of the cells over the time course of cell culture was tested. As expected, the stem cell markers NANOG and POUF5A1 were highly expressed on day −1 and day 0, but by day 3 after neural induction, the expression of these markers was significantly reduced. In contrast, SOX2, which is both a marker of stem cells and a NPC marker, did not show any decrease at these time points, but reached maximal expression at day 15 along with other neuroectoderm and NPC markers. These data are consistent with the known proliferative role of FGF-2. Most neuronal structural markers (MAP2, MAPT, NFASC, TUBA1A) were significantly increased from day 28 to 40. Once ne...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com