Core-shell structure nano particle and preparation method thereof
A nanoparticle, core-shell structure technology, which can be used in pharmaceutical formulations, medical preparations with non-active ingredients, and inorganic non-active ingredients, etc., and can solve problems such as the inability to achieve effective controlled release of active drugs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
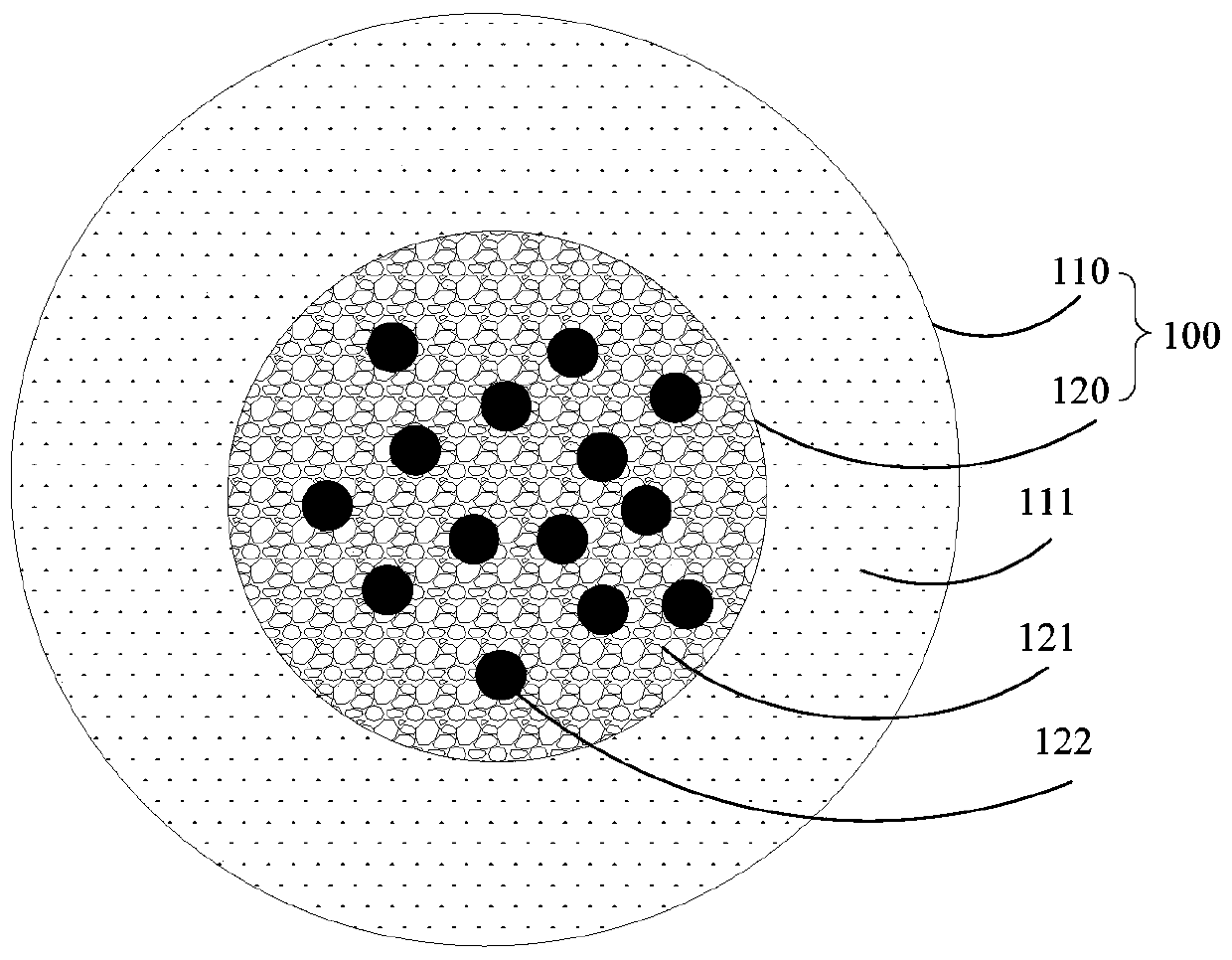
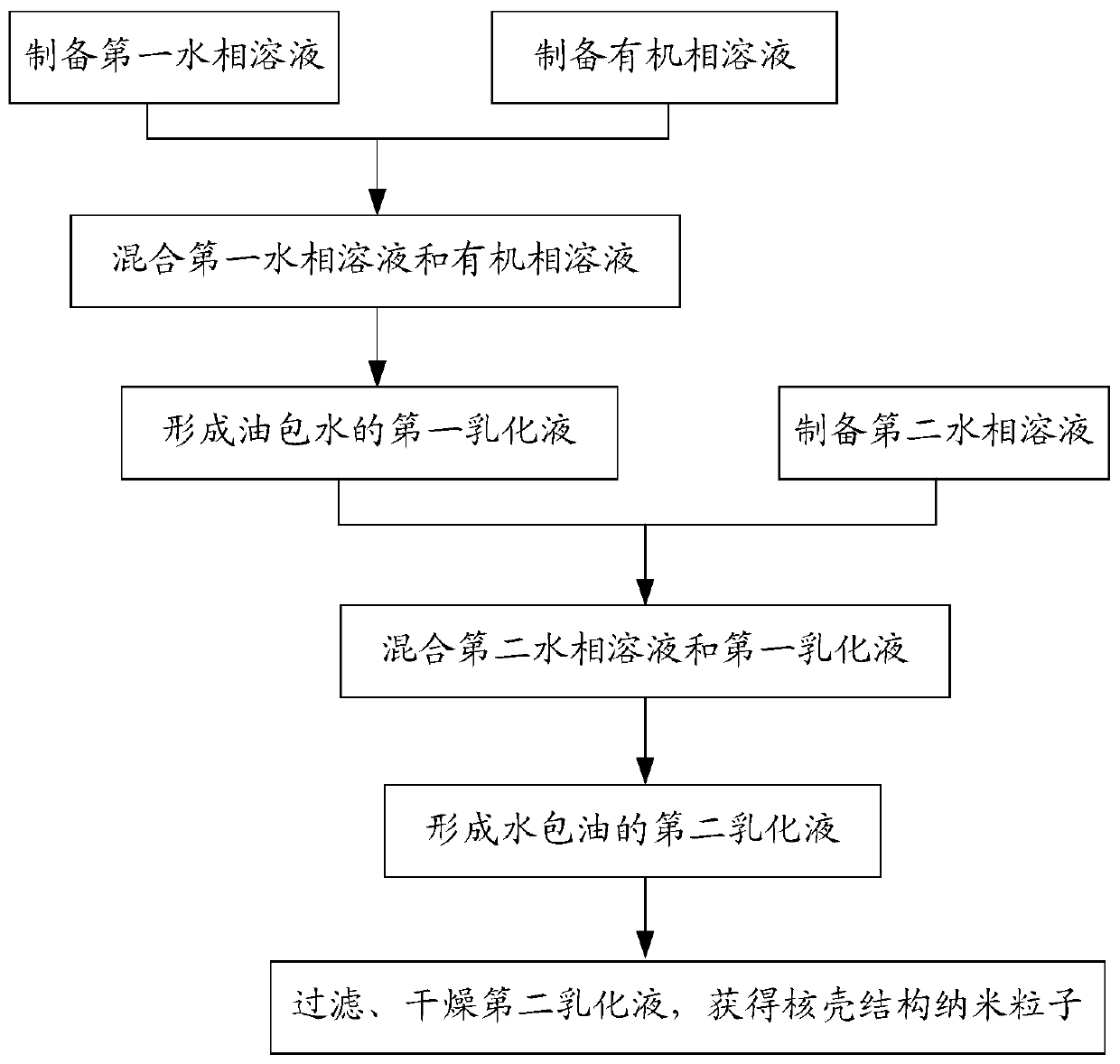
[0042] see figure 2 , the present invention also proposes a method for preparing nanoparticles with a core-shell structure, including the nanoparticles with the core-shell structure. The nanoparticles with the core-shell structure refer to the above-mentioned embodiments. All the technical solutions at least have all the beneficial effects brought by the technical solutions of the above embodiments, and will not be described here one by one. The preparation method of the core-shell nanoparticle comprises the following steps:
[0043] preparing a first aqueous phase solution, the first aqueous phase solution comprising a water-soluble substance and an active drug;
[0044] preparing an organic phase solution, the organic phase solution includes a fat-soluble polymer;
[0045] mixing the first aqueous phase solution and the organic phase solution to form a first water-in-oil emulsion;
[0046] preparing a second aqueous phase solution, mixing the second aqueous phase solution ...
Embodiment 1
[0061] A method for preparing nanoparticles with a core-shell structure, comprising the following steps: first, mixing ethyl orthosilicate, ethyl silicate, water and hydrochloric acid solution at normal temperature, so that ethyl orthosilicate and ethyl silicate Esters were hydrolyzed to produce a clear silicic acid solution; then, antibiotics were dissolved into the silicic acid solution to prepare the first aqueous phase solution (S1); then, 1 volume of the first aqueous phase solution (S1) was added to 5 volumes of In the dichloromethane solution of polylactic acid with a concentration of 20mg / ml, ultrasonic emulsification for 1 minute to obtain the first emulsion (R1); then add 1 volume of the first emulsion (R1) to 2 volumes of the second aqueous phase solution In (S2), the second emulsion (R2) was obtained by ultrasonic emulsification for 2 minutes; finally, the second emulsion (R2) was stirred for 4 hours, filtered and dried to obtain nanoparticles with core-shell struct...
Embodiment 2
[0064] A method for preparing nanoparticles with a core-shell structure, comprising the following steps: first, dissolving chitosan in a weak acid to obtain a chitosan aqueous solution with a mass fraction of 1%; then, dissolving antibiotics into the above-mentioned chitosan aqueous solution, Prepare the first aqueous phase solution (S1); then, add 1 volume of the first aqueous phase solution (S1) to 5 volumes of a dichloromethane solution of polyethylene glycol-lactic-co-glycolic acid copolymer with a concentration of 30 mg / ml In the process, ultrasonic emulsification for 2 minutes to obtain the first emulsion (R1); then add 1 volume of the above-mentioned first emulsion (R1) to 4 volumes of the second aqueous phase solution (S2), and ultrasonic emulsification for 1 minute to obtain the second emulsification solution (R2); finally, the above-mentioned second emulsion (R2) was stirred for 4 hours, filtered and dried to obtain core-shell structure nanoparticles. Wherein, in the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com