A kind of preparation method of tetrahydrophenazine derivative
A technology of tetrahydrophenazine and derivatives, which is applied in the field of preparation of tetrahydrophenazine derivatives, can solve the problems of high price of phenazine compounds, difficult commercialization, expensive reagents, etc., and achieves low catalyst usage and raw materials. Non-toxic, good compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043]
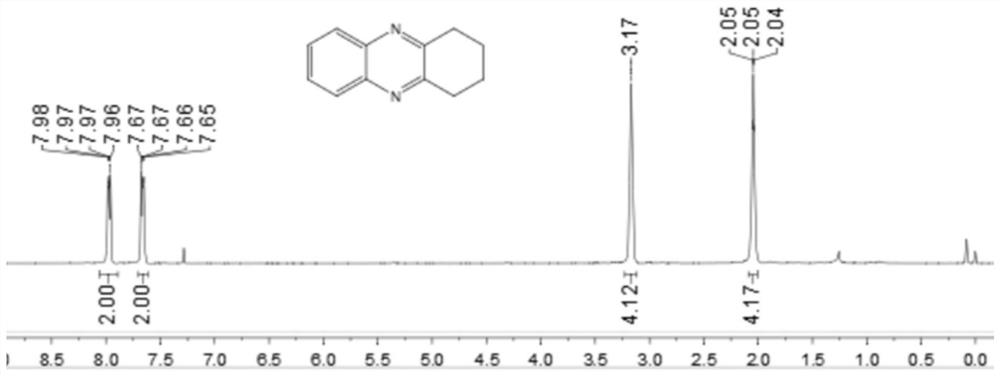
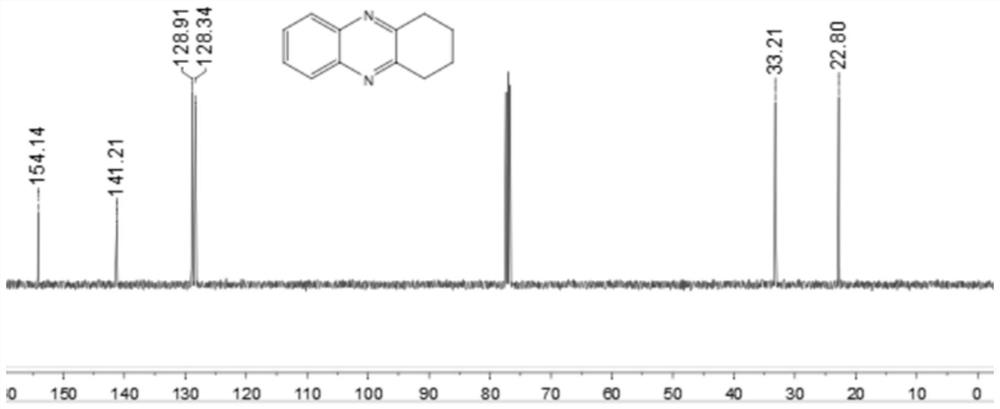
[0044] Add 0.25 mmol 2-nitroaniline, 0.35 mmol o-diphenol, 0.0075 mmol palladium carbon, 0.05 mmol potassium carbonate, 1.5 ml p-xylene to a schlenk tube, at 120 °C, H 2 Under conditions (the hydrogen pressure used is one atmospheric pressure) after stirring and reacting for 12 hours, stop heating and stirring, cool to room temperature, remove the solvent by rotary evaporation under reduced pressure, and then separate and purify by column chromatography to obtain the target product. The volume ratio of the deliquified petroleum ether: ethyl acetate mixed solvent is 15:1, and the yield is 74%.
[0045] of the resulting product 1 H-NMR spectrum and 13 The C-NMR spectra are as follows figure 1 and figure 2 As shown, the structural characterization data are as follows: 1 H NMR (400MHz, CDCl 3): δ7.97(dd, J=6.4, 3.2Hz, 2H), 7.66(dd, J=6.0, 3.2Hz, 2H), 3.17(s, 4H), 2.09-2.01(m, 4H). 13 C NMR (101MHz, CDCl 3 ): δ154.14, 141.21, 128.91, 128.34, 33.21, 22.80. IR(KB...
Embodiment 2
[0047]
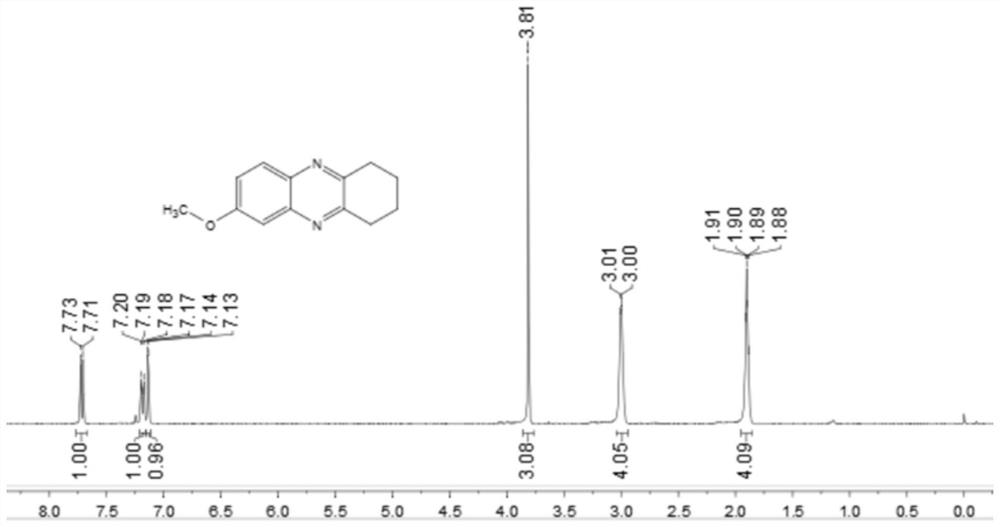
[0048] Add 0.25 mmol 4-methoxy-2-nitroaniline, 0.35 mmol o-diphenol, 0.0075 mmol palladium carbon, 0.1 mmol potassium carbonate, 1.5 ml p-xylene to a schlenk tube at 120 °C, H 2 Under conditions (the hydrogen pressure used is one atmospheric pressure) after stirring and reacting for 12 hours, stop heating and stirring, cool to room temperature, remove the solvent by rotary evaporation under reduced pressure, and then separate and purify by column chromatography to obtain the target product. The volume ratio of the deliquified petroleum ether:ethyl acetate mixed solvent is 8:1, and the yield is 85%.
[0049] of the resulting product 1 H-NMR spectrum and 13 The C-NMR spectra are as follows image 3 and Figure 4 As shown, the structural characterization data are as follows: 1 H NMR (400MHz, CDCl 3 ): δ7.72(d, J=9.1Hz, 1H), 7.19(dd, J=9.1, 2.7Hz, 1H), 7.14(d, J=2.7Hz, 1H), 3.81(s, 3H), 3.00 (d, J=4.2Hz, 4H), 1.90 (dd, J=6.5, 3.0Hz, 4H). 13 C NMR (101MHz, CDCl ...
Embodiment 3
[0051]
[0052] Add 0.25 mmol 5-(4-methylpiperazine)-2-nitroaniline, 0.5 mmol catechol, 0.015 mmol palladium hydroxide on carbon, 0.05 mmol cesium carbonate, 1.5 ml toluene to a schlenk tube, at 110°C, H 2 After stirring and reacting for 16 hours under conditions (the hydrogen pressure used is one atmospheric pressure), stop heating and stirring, cool to room temperature, remove the solvent by rotary evaporation under reduced pressure, and then separate and purify by column chromatography to obtain the target product. The volume ratio of the deliquified petroleum ether:ethyl acetate mixed solvent is 6:1, and the yield is 80%.
[0053] of the resulting product 1 H-NMR spectrum and 13 The C-NMR spectra are as follows Figure 5 and Image 6 As shown, the structural characterization data are as follows: 1 H NMR (400MHz, CDCl 3 ): δ7.73(d, J=9.3Hz, 1H), 7.35(dd, J=9.3, 2.4Hz, 1H), 7.12(d, J=2.4Hz, 1H), 3.35-3.27(m, 4H) , 3.01 (d, J=2.0Hz, 4H), 2.57-2.49 (m, 4H), 2.28 (s, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com