Buccal tablets
A technology for buccal tablets and exosomes, which is applied to buccal tablets. It can solve the problems of slow effect of buccal tablets and slow melting of buccal tablets, and achieve good application value, low cost and good whitening effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
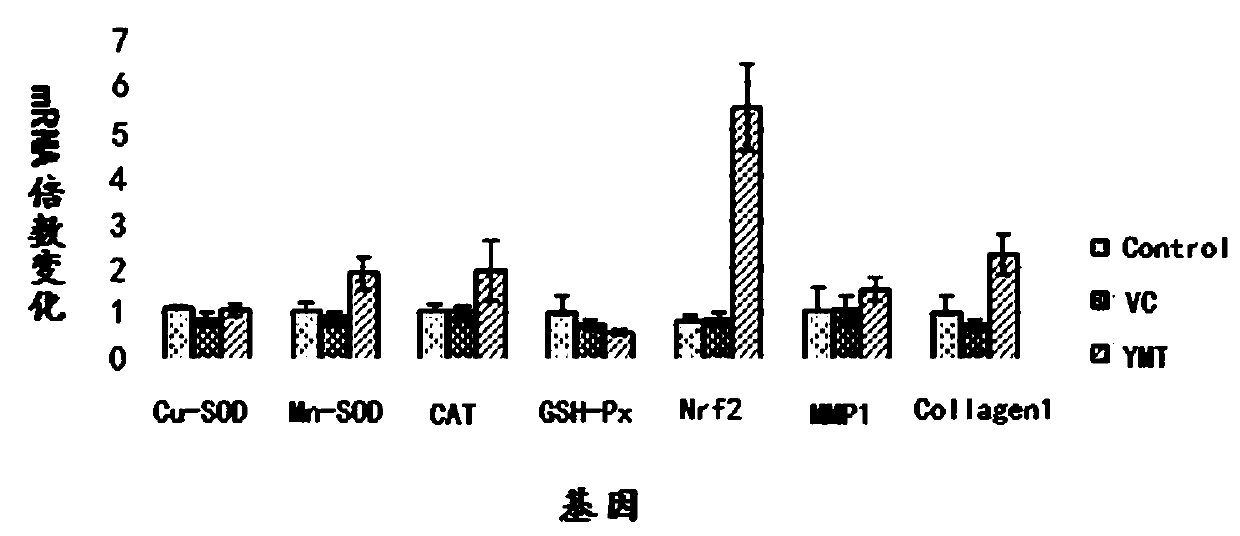
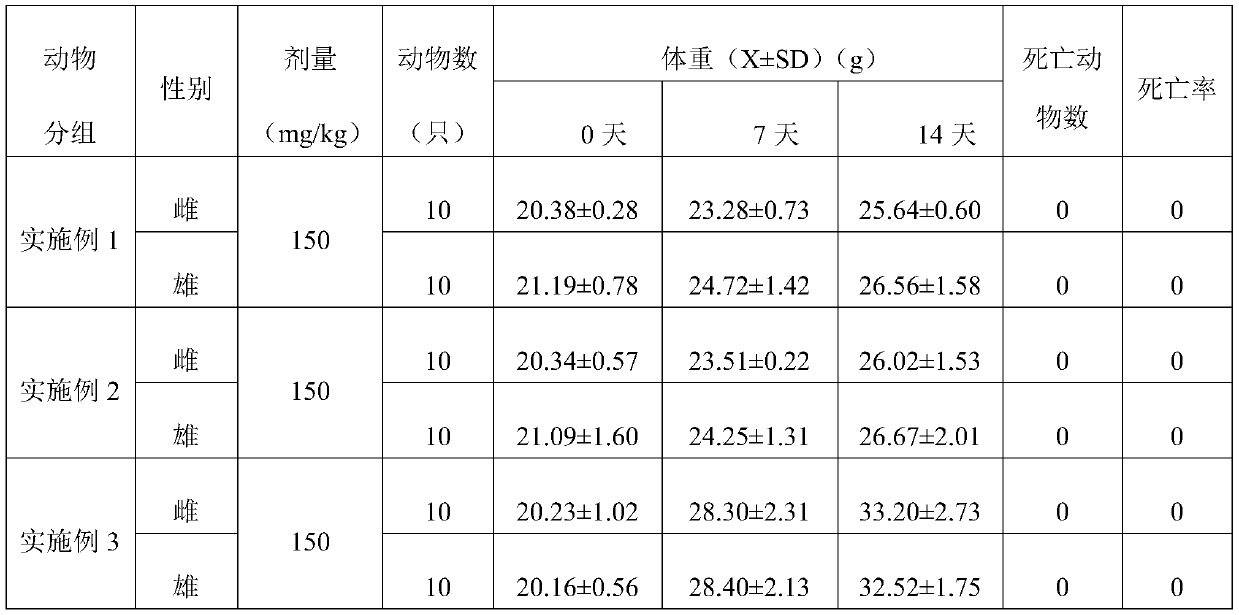
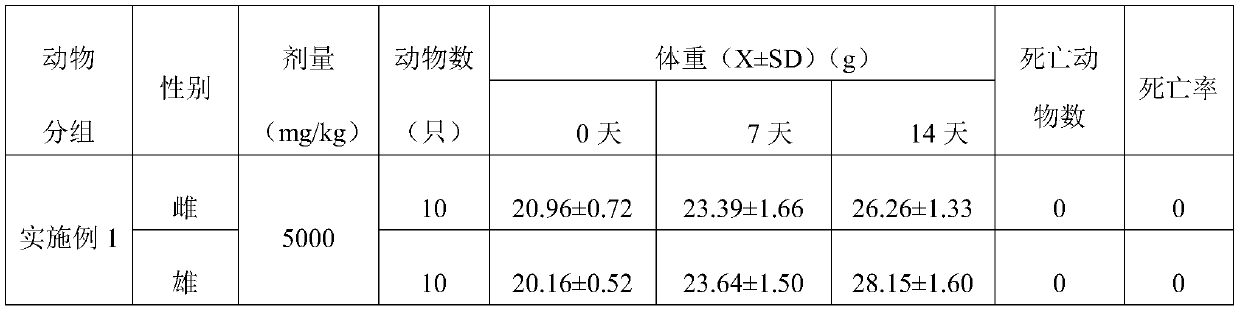
[0060] The present embodiment provides a buccal tablet, according to parts by weight, the buccal tablet comprises:
[0061] 5 parts lemon exosomes, 5 parts vitamin C, 5 parts yeast extract, 0.1 parts stearic acid, 10 parts hydroxyproline, 5 parts pectin and 0.5 parts lemon fruit powder.
[0062] The preparation method of present embodiment buccal tablet is as follows:
[0063] S1. Add 5 parts of lemon exosomes, 5 parts of vitamin C, 5 parts of yeast extract, 0.5 parts of lemon fruit powder, 0.1 parts of stearic acid and 10 parts of hydroxyproline into 5 parts of fully dissolved pectin, form a homogeneous solution;
[0064] S2. degassing the solution obtained in step S1;
[0065] S3. injecting the solution degassed in step S2 into the mould;
[0066] S4. pre-freezing the mold filled with the solution in step S3 at a temperature of -150° C. for 1 min;
[0067] S5. Transfer the mold pre-frozen in step S3 into a freeze dryer, and freeze-dry it for 1 hour under the conditions o...
Embodiment 2
[0069] The present embodiment provides a buccal tablet, according to parts by weight, the buccal tablet comprises:
[0070] 20 parts lemon exosomes, 10 parts vitamin C, 10 parts yeast extract, 5 parts stearic acid, 20 parts hydroxyproline, 10 parts pectin and 2 parts lemon fruit powder.
[0071] The preparation method of present embodiment buccal tablet is as follows:
[0072] S1. Add 20 parts of lemon exosomes, 10 parts of vitamin C, 10 parts of yeast extract, 2 parts of lemon fruit powder, 5 parts of stearic acid and 20 parts of hydroxyproline into 10 parts of fully dissolved pectin, form a homogeneous solution;
[0073] S2. degassing the solution obtained in step S1;
[0074] S3. injecting the solution degassed in step S2 into the mould;
[0075] S4. Prefreezing the mold filled with the solution in step S3 for 60 minutes at a temperature of -20°C;
[0076] S5. Transfer the pre-frozen mold in step S3 into a freeze dryer, and freeze-dry for 5 hours under the conditions of...
Embodiment 3
[0078] The present embodiment provides a buccal tablet, according to parts by weight, the buccal tablet comprises:
[0079] 10 parts lemon exosomes, 6 parts vitamin C, 7 parts yeast extract, 1 part stearic acid, 12 parts hydroxyproline, 6 parts pectin and 1 part lemon fruit powder.
[0080] The preparation method of present embodiment buccal tablet is as follows:
[0081] S1. Add 10 parts of lemon exosomes, 6 parts of vitamin C, 7 parts of yeast extract, 1 part of lemon fruit powder, 1 part of stearic acid and 12 parts of hydroxyproline into 6 parts of fully dissolved pectin, form a homogeneous solution;
[0082] S2. degassing the solution obtained in step S1;
[0083] S3. injecting the solution degassed in step S2 into the mould;
[0084] S4. Prefreezing the mold filled with the solution in step S3 for 10 minutes at a temperature of -50° C.;
[0085] S5. Transfer the mold pre-frozen in step S3 into a freeze dryer, and freeze-dry for 2 hours under the conditions of a press...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com