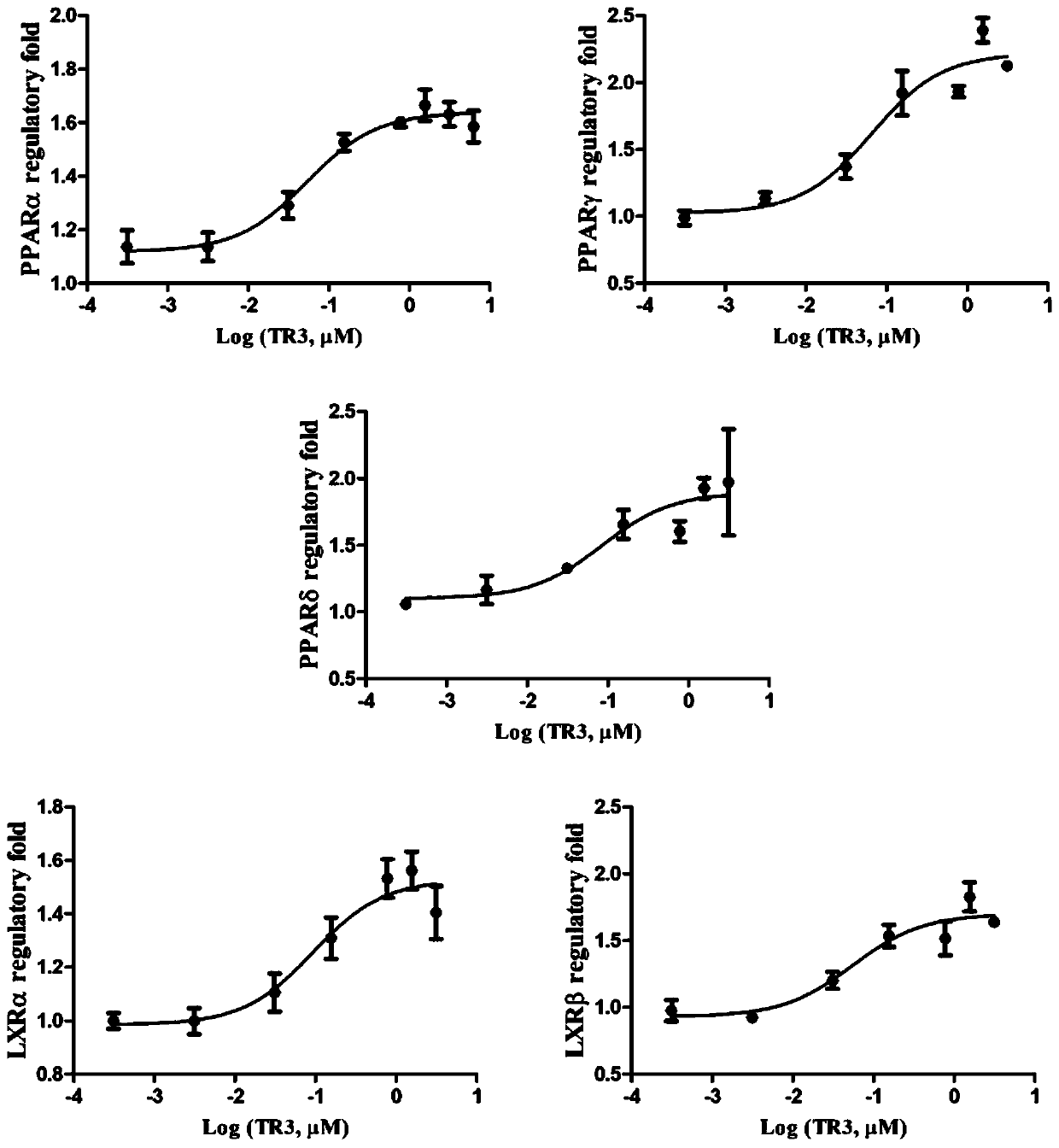
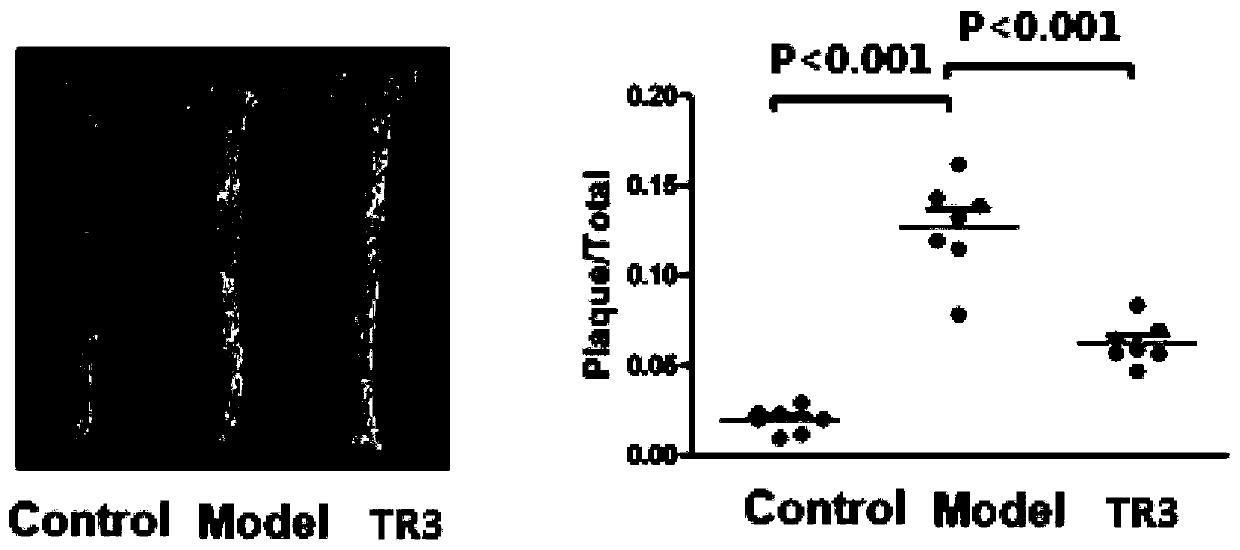
Tetrahydroindoloquinazoline compounds and application thereof
A technology of indoloquinazolines and compounds, applied in the field of tetrahydroindoloquinazolines, capable of solving problems such as low oral bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
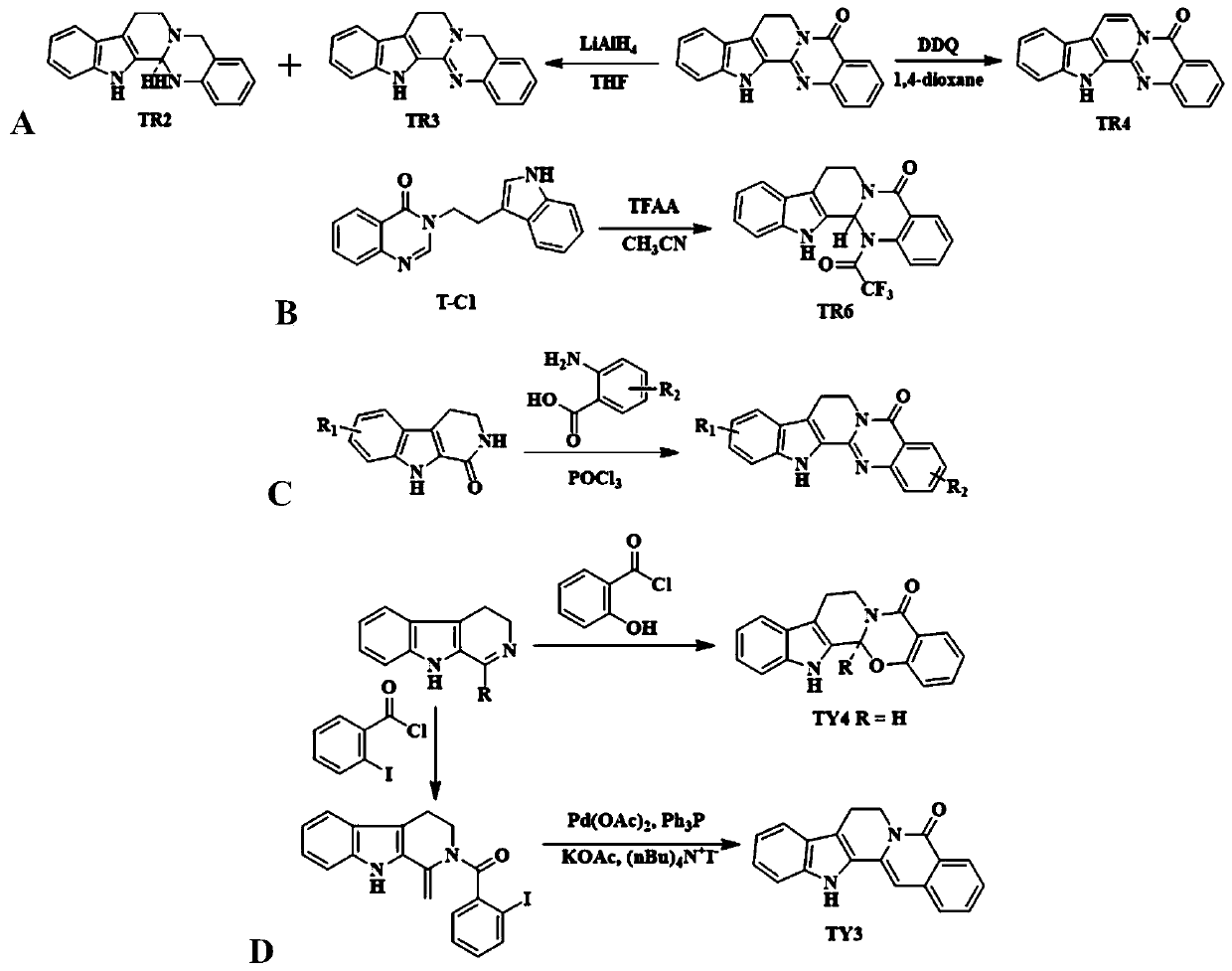
[0070] Embodiment 1: the synthesis of TR2 and TR3
[0071] Add evodiamine (2.0g, 7mmol) into 100mL dry THF, slowly add lithium aluminum hydride (1.06g, 28mmol) in batches at room temperature, and stir at room temperature for 8h after the addition (the reaction system starts to turn yellow, The color gradually darkens to green, accompanied by fever).
[0072] Quench the reaction with 1M hydrochloric acid, filter with suction and wash with DCM, CH 3 OH washed, and the filtrate was collected. After most of the solvent was evaporated from the filtrate, the pH was adjusted to 8-9, extracted with DCM and dried. The filtrate and the extract were combined and concentrated, and separated by silica gel column chromatography using AcOEt:PE=1:4 as the eluent to obtain pure products TR2 and TR3 respectively.
[0073] 5,7,8,13,13b,14-Hexahydroindolo[2’,3’:3,4]pyrido[2,1-b]quinazoline (TR2)
[0074]
[0075] TR2 (16 mg, 3% relative yield), mp 181-183°C. MS(ESI m / z)276.21(M+H) + , 1...
Embodiment 2
[0079] Embodiment 2: the synthesis of indolo[2',3':3,4][2,1-b]quinazolin-5(13H)-ketone (TR4)
[0080]
[0081] At 50°C, dissolve evodiamine (1.44g, 5mmol) in 1,4-dioxane (20mL), and add dropwise DDQ (1.14g, 5mmol) in 1,4-dioxane (20mL) at 80°C. Oxycycline solution (red), the reaction system immediately turned green, and after reflux for 4 hours, the solvent was distilled off. Wash with 10% potassium hydroxide solution several times to remove DDQ-2H in the system, and after filtration, wash with 10% aqueous hydrochloric acid solution and pure water successively, and dry with suction to obtain the crude product. The crude product was washed with dichloromethane to give TR4 (0.67 g, 47% yield), a pale yellow solid, mp 282-284°C. MS(ESI,m / z)286.17(M+H) + , 318.30(M+Na) + .
[0082] 1 H NMR (400MHz, DMSO-d6) δ12.71(s, 1H, 13-NH), 8.64(d, J=7.6Hz, 1H, 4-H), 8.38(dd, J=1.2, 8.0 Hz, 1H ,9-H),8.18(d,J=8.0Hz,1H,7-H),7.94(td,1H,J=1.6,7.6Hz,2-H),7.86-7.84(m,2H,1, 3-2H),7.69 (d,J...
Embodiment 3
[0083] Example 3: 14-trifluoroacetyl-8,13,13b,14-dihydroindolo[2',3':3,4][2,1-b]quinazoline-5(7H )-Kone (TR6) Synthesis
[0084] 3-(2-(1H-3-indole)ethyl)-3H-quinazolin-4-one (1.45g, 5mmol) was suspended in 30mL of acetonitrile, and the ), a solution of trifluoroacetic anhydride (0.7mL, 5mmol) in acetonitrile was added dropwise, and the reaction system immediately became a transparent solution. The reaction continued for 2h, and a white solid precipitated out. The white solid was obtained by filtration, and the pure product TR6 was obtained after separation by silica gel column chromatography using AcOEt:PE=1:4 as the eluent.
[0085]
[0086] TR6 (0.63g), mp 199-201°C. HRMS(ESI m / z)calcd[M+H] + for C 20 h 13 o 2 N 3 f 3 384.1033 found 385.1637.
[0087] 1 H NMR (500MHz, DMSO-d6) δ11.41(s,1H,13-NH),7.90(d,J=7.5Hz,1H,4-H),7.67(t,J=7.5Hz, 1H,3 -H),7.38(t,J=7.5Hz,2H,2,9-2H),7.29(d,J=8.0Hz,1H,12-H),7.09(td,J=1.0,7.5Hz,2H ,10,11-H), 6.98(td,J=1.0,7.5Hz,1H,1-H),4.71(dd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com