Novel proline 3-hydroxylase and application thereof
A proline, hydroxylase technology, applied in the biological field, can solve the problems of low industrial production, low selectivity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment 1. Synthesis and clone expression of proline 3-hydroxylase
[0064] First, the inventor synthesized the genes shown in the sequences SEQ ID NO: 1 and SEQ ID NO: 3 through the whole gene (the encoded amino acid sequences are SEQ ID NO: 2 and 4, respectively), and respectively adopted the Nde I inner Dicer site, the 3' end was connected to the Gaokaobei plasmid pET21a using the Hind III endonuclease site, and the obtained recombinant plasmids were named pET21a-Ubp4h and pET21a-Amp3h, and then the recombinant plasmids pET21a-Ubp4h, pET21a-Ubp4h, pET21a-Amp3h was introduced into Escherichia coli BL21a(DE3) competent cells to obtain recombinant Escherichia coli BL21a(DE3)(pET21a-Ubp4h) and BL21a(DE3)(pET21a-Amp3h). Proline 3-hydroxylase was induced to express as follows.
[0065] Take 5 μl of glycerol bacteria solution and inoculate 5ml of LB medium (10g / l peptone, 10g / l NaCl, 5g / l yeast extract) containing antibiotics (100μg / ml ampicillin), and culture overnight...
Embodiment 2
[0066] Embodiment 2. Enzyme activity assay of proline 3-hydroxylase
[0067] The cultured BL21a(DE3)(pET21a-Ubp4h) and BL21a(DE3)(pET21a-Amp3h) cells were centrifuged at 4°C and 7000rpm for 6min to collect the cells, and then washed with 100mM Tris-HCl, 100mM NaCl, 10% glycerol , pH 7.4 buffer solution to wash the cells three times, break up immediately or store in a -80°C refrigerator for later use. The collected cells were resuspended with an appropriate amount of buffer solution of 50 mM MES, 100 mM NaCl, 10% glycerol, pH 6.5 and then sonicated. The cell lysate was centrifuged at 4°C and 8000rpm for 35min, then the supernatant was transferred to a pre-cooled centrifuge tube, and then His SpinTrap was used to TM columns (manufactured by GE Healthcare) nickel column to purify the protein and elute the impurity protein with concentration gradient imidazole, after removing the imidazole to obtain the pure enzyme of proline-3-hydroxylase, aliquot into pre-cooled small centrifug...
Embodiment 3
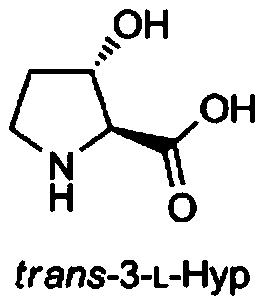
[0074] Embodiment 3. Catalyzed production of trans-3-hydroxyl-L-proline with L-proline as substrate
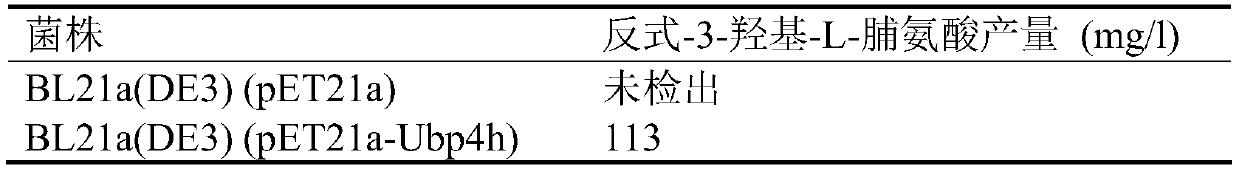
[0075] First, as described in Example 1, the strain BL21a(DE3)(pET21a-Ubp4h) was cultured and induced to express in shake flasks, and the above-mentioned cultured cells were centrifuged at 4°C and 7000rpm for 6min to collect the cells, and then 100mM Tris- Wash the cells three times with HCl, 100mM NaCl, 10% glycerol, pH 7.4 buffer and collect the cells. Suspend the collected cells with 10ml reaction buffer / 100ml shaker flask and culture them in a shaker at 30°C for 24h. The reaction buffer contains 50mM ES (pH 6.5), 0.5mM FeSO 4 , 1.5mM ascorbic acid, 20mM L-Pro, 14mM α-ketoglutarate. After the reaction, centrifuge at 4°C and 7000rpm for 7 minutes to separate the supernatant, and transfer the supernatant to a clean centrifuge tube for detection of the production of trans-3-hydroxyl-L-proline. The blank used in this example The control strain was BL21a(DE3)(pET21a). Detect ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com