Melatonin (MT1/MT2) receptor stimulant, and preparation method and application thereof
A halogen and optical isomer technology, applied in the field of medicinal chemistry, pharmacology, and pharmacology, can solve the problems of weak experimental activity, weak clinical effect, and insufficient distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
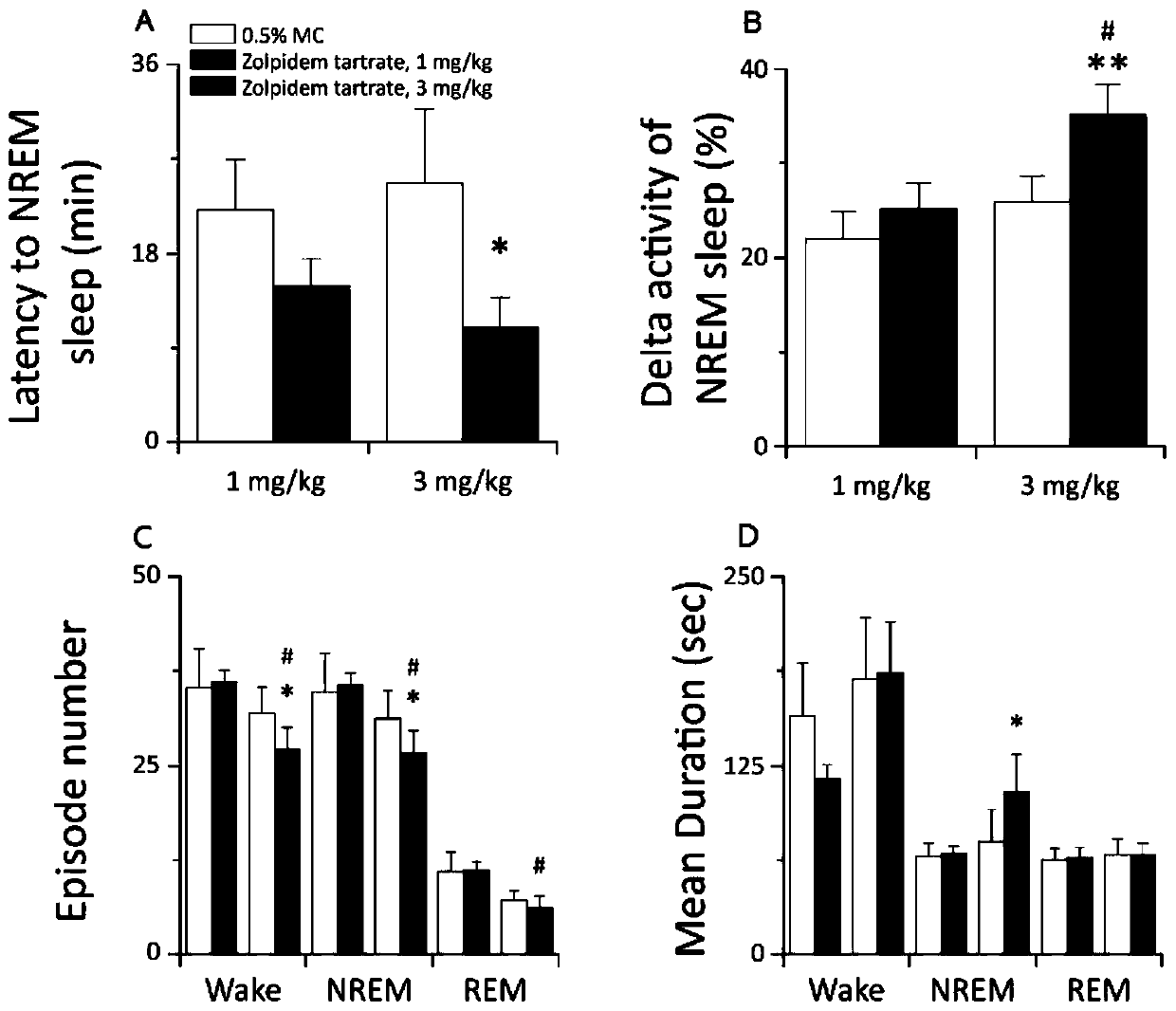
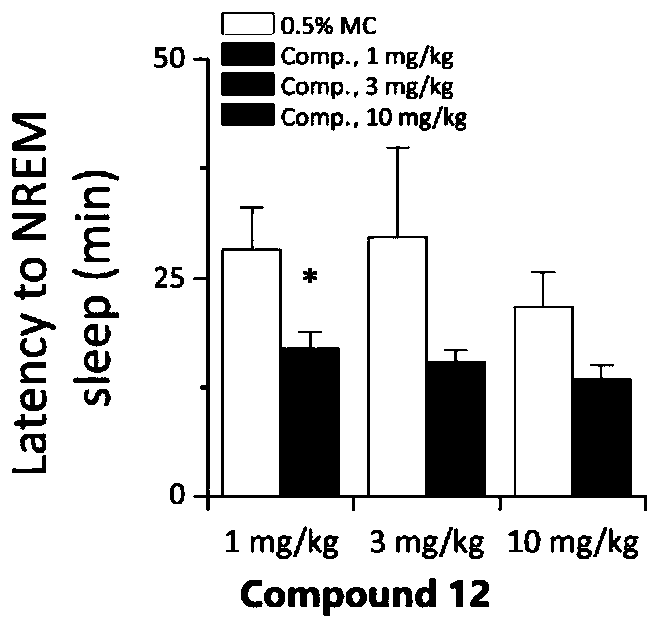
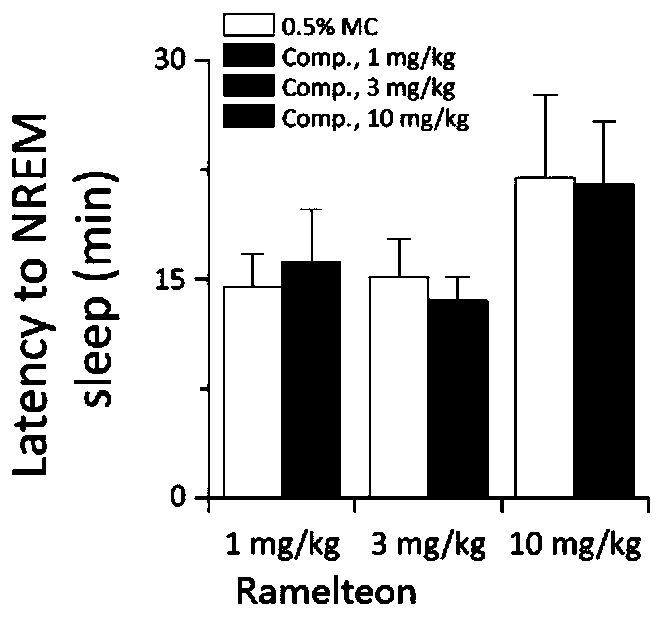
Image
Examples
Embodiment 1
[0182] (S)-3-(4,5-difluoro-1,2,7,8-tetrahydrobenzo[1,2-b:4,3-b']difuran-1-yl)-N- The preparation of methyl propionamide (formula 1 compound)
[0183]
[0184] Step (1) Synthesis of 2,3-difluorophenyl-2-chloroacetate (compound of formula 1-2).
[0185] The compound of formula 1-1 (2.6g, 0.02mol) was dissolved in dry dichloromethane, ice bathed, triethylamine (2.63g, 0.026mol) was added, and chloroacetyl chloride (2.94g, 0.026mol, 1.3eq), dropwise, naturally warming up to room temperature overnight, thin-layer chromatography detection, the reaction is completed, poured into ice water to quench, dichloromethane extraction, organic phase washed with saturated brine, dried over anhydrous sodium sulfate, filtered, reduced The solvent was evaporated under pressure to obtain a brown liquid (4.13 g, 100%), namely the compound of formula 1-2, which was directly put into the next reaction.
[0186] Step (2) Synthesis of 2-chloro-1-(3,4-difluoro-2-hydroxyphenyl)ethan-1-one (compound ...
Embodiment 2
[0227] (S)-3-(4,5-difluoro-1,2,7,8-tetrahydrobenzo[1,2-b:4,3-b']difuran-1-yl)-N- The preparation of propyl propionamide (formula 2 compound)
[0228]
[0229] (S)-3-(4,5-difluoro-1,2,7,8-tetrahydrobenzo[1,2-b:4,3-b']difuran-1-yl)-N- Synthesis of Propylpropionamide (Compound of Formula 2). .
[0230] The compound of formula 1-15 (55 mg, 0.2 mmol) was dissolved in dry dichloromethane (5 mL), and HOBT (42 mg, 0.3 mmol), HATU (117 mg, 0.3 mmol), diisopropylethylamine (35 mg, 0.5mmol), n-propylamine (18mg, 0.3mmol), room temperature reaction overnight, thin-layer chromatographic detection, after the reaction was completed, the solvent was evaporated under reduced pressure, and column chromatography (EA) gave a white solid (28mg, 45%) formula 2 compound .
[0231] Formula 2 compound: 1 H NMR (400MHz, Chloroform-d)δ5.38(s,1H),4.73–4.55(m,3H),4.33(dd,J=9.0,5.2Hz,1H),3.59–3.46(m,1H), 3.15(dddtd,J=18.0,15.3,12.9,7.7,5.7Hz,4H),2.20–2.02(m,3H),1.98–1.84(m,1H),1.47(d,J=7.3Hz,2H), ...
Embodiment 3
[0233] (S)-3-(4,5-difluoro-1,2,7,8-tetrahydrobenzo[1,2-b:4,3-b']difuran-1-yl)-N- The preparation of isopropyl propionamide (formula 3 compound)
[0234]
[0235] (S)-3-(4,5-difluoro-1,2,7,8-tetrahydrobenzo[1,2-b:4,3-b']difuran-1-yl)-N- Synthesis of isopropylpropionamide (compound of formula 3). .
[0236] The compound of formula 1-15 (41 mg, 0.15 mmol) was dissolved in dry dichloromethane (5 mL), and HOBT (32 mg, 0.225 mmol), HATU (88 mg, 0.225 mmol), diisopropylethylamine (26 mg, 0.375mmol), isopropylamine (14mg, 0.225mmol), react overnight at room temperature, thin-layer chromatography detects, after the reaction is completed, the solvent is evaporated under reduced pressure, and column chromatography (EA) obtains a white solid (20mg, 42%), the formula 3 compounds.
[0237] Formula 3 compound: 1 H NMR (400MHz, Chloroform-d) δ5.21(s,1H),4.72–4.52(m,3H),4.32(dd,J=9.0,5.2Hz,1H),3.99(dp,J=7.7,6.5 Hz,1H),3.50(dd,J=8.6,4.5Hz,1H),3.29–3.16(m,1H),3.09(dddd,J=15.3,9.2,6.9,1....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com