Water-in-oil-system breast enhancement polypeptide composition
A technology of polypeptide composition and water-in-oil, which is applied in the direction of cosmetics, cosmetic preparations, dressing preparations, etc., and can solve the problems of rising costs, harm, and increasing the amount of input
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
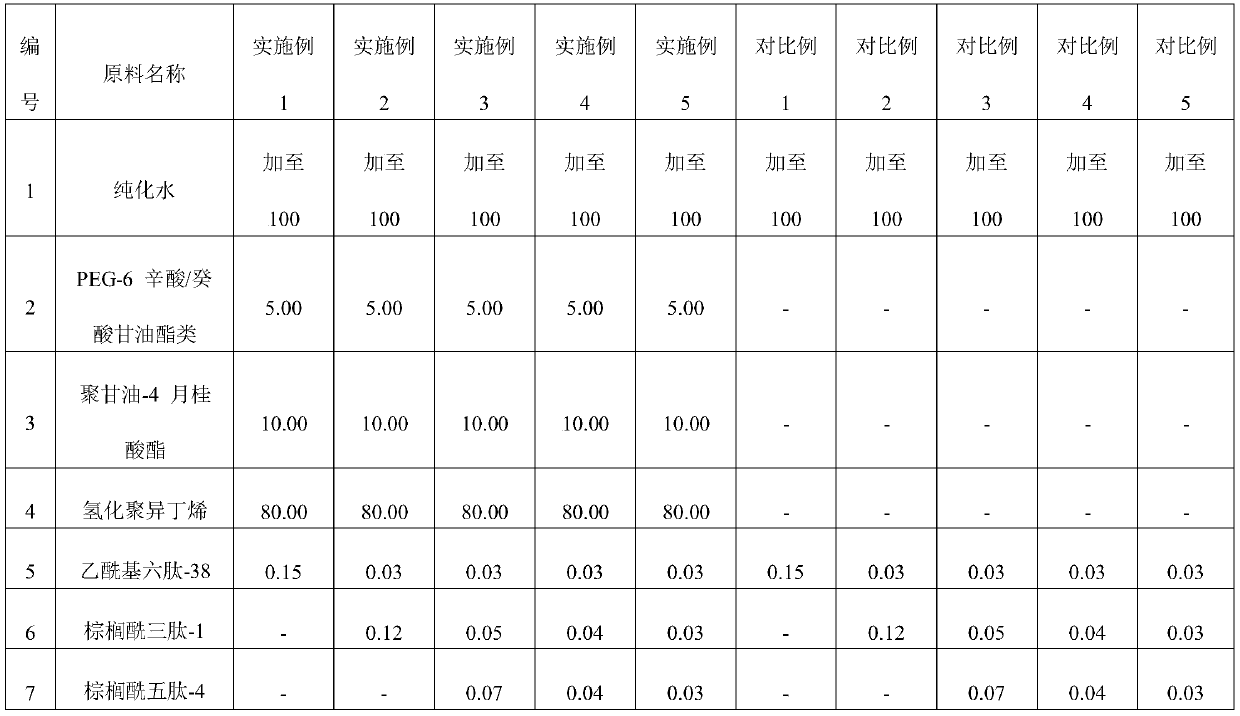
Embodiment 1-5 and comparative example 1-5
[0053] The preparation method of embodiment 1-5 and comparative example 1-5 composition:
[0054] 1. Accurately weigh raw materials 5, 6, 7, 8, and 9, add the prescribed amount of raw material 1, and fully dissolve;
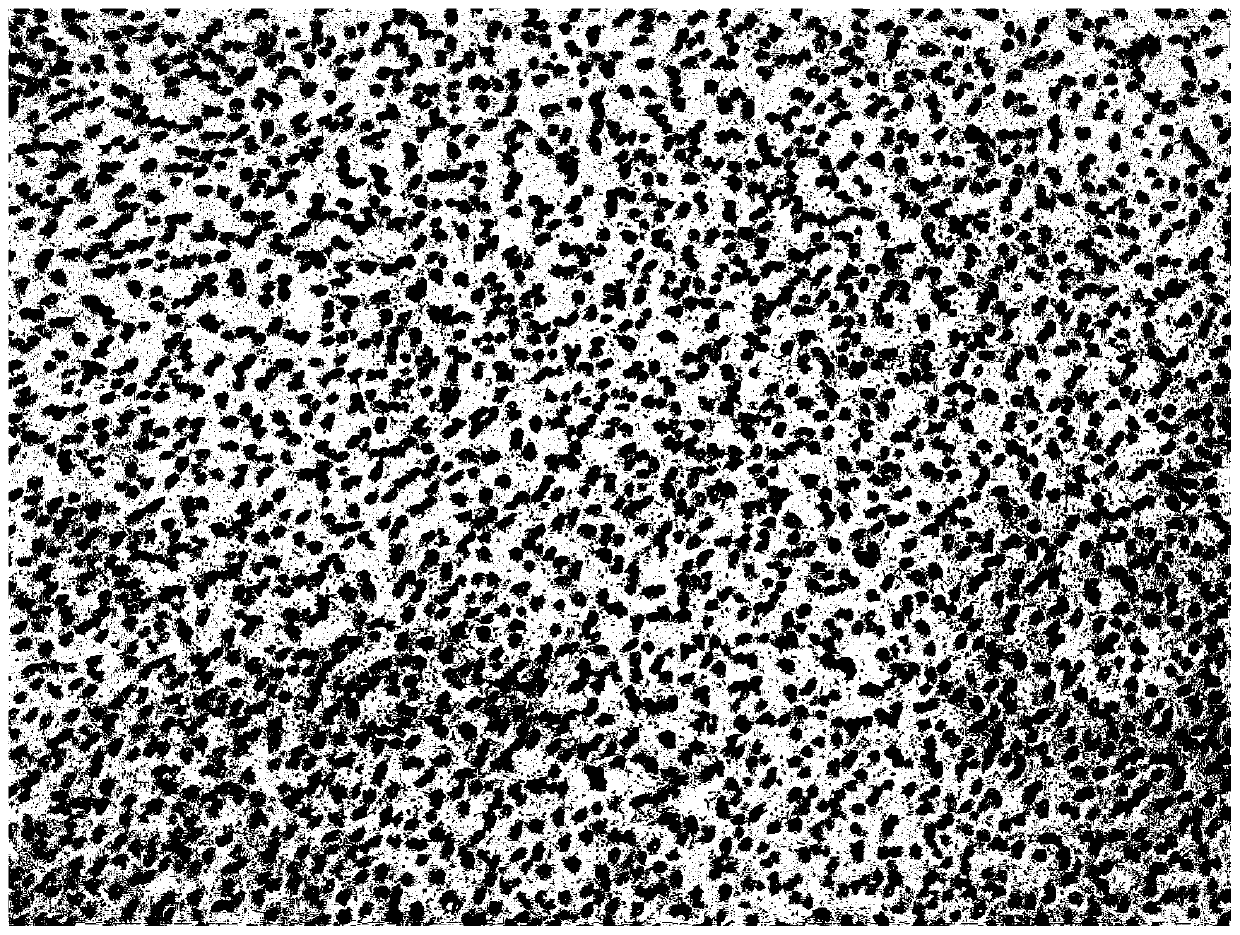
[0055] 2. Accurately weigh raw materials 2, 3, and 4, and mix them with the solution obtained in step 1 to get ready. Adopt biological digital microscope to observe the microscopic image of embodiment 2, the results are shown in figure 1 , Many small droplets of uniform size were observed under the microscope at 40*10 times, evenly distributed in the oil phase, forming a water-in-oil system.
Embodiment 1-5、 comparative example 1-5
[0061] 1.3 Test basis
[0062] "Chinese Pharmacopoeia" 2015 Edition Four General Rules 9001 Guiding Principles for Stability Testing of APIs and Preparations
[0063] 1.4 Test conditions and inspection items
[0064] Accelerated test: Constant temperature and humidity chamber at 40°C±2°C, RH75%±5%, respectively, at the first, second, third, and sixth month, detect the peptide content in each sample by HPLC to evaluate its stability.
[0065] Long-term test: Constant temperature and humidity chamber at 25°C±2°C, RH60%±10%, at the 3rd, 6th, 9th, 12th, 18th, 24th, and 36th month respectively, detect the peptide content in each sample by HPLC to evaluate its stability.
[0066] 1.5 Stability test results
[0067] After the samples of Examples 1-5 and Comparative Examples 1-5 were placed under accelerated test conditions for 6 months, the stability data are shown in the following table 1:
[0068] Table 1 accelerates the stability test data of 6 months (content should be 95%-10...
Embodiment 1、 comparative example 1
[0080] 2.3 Test method
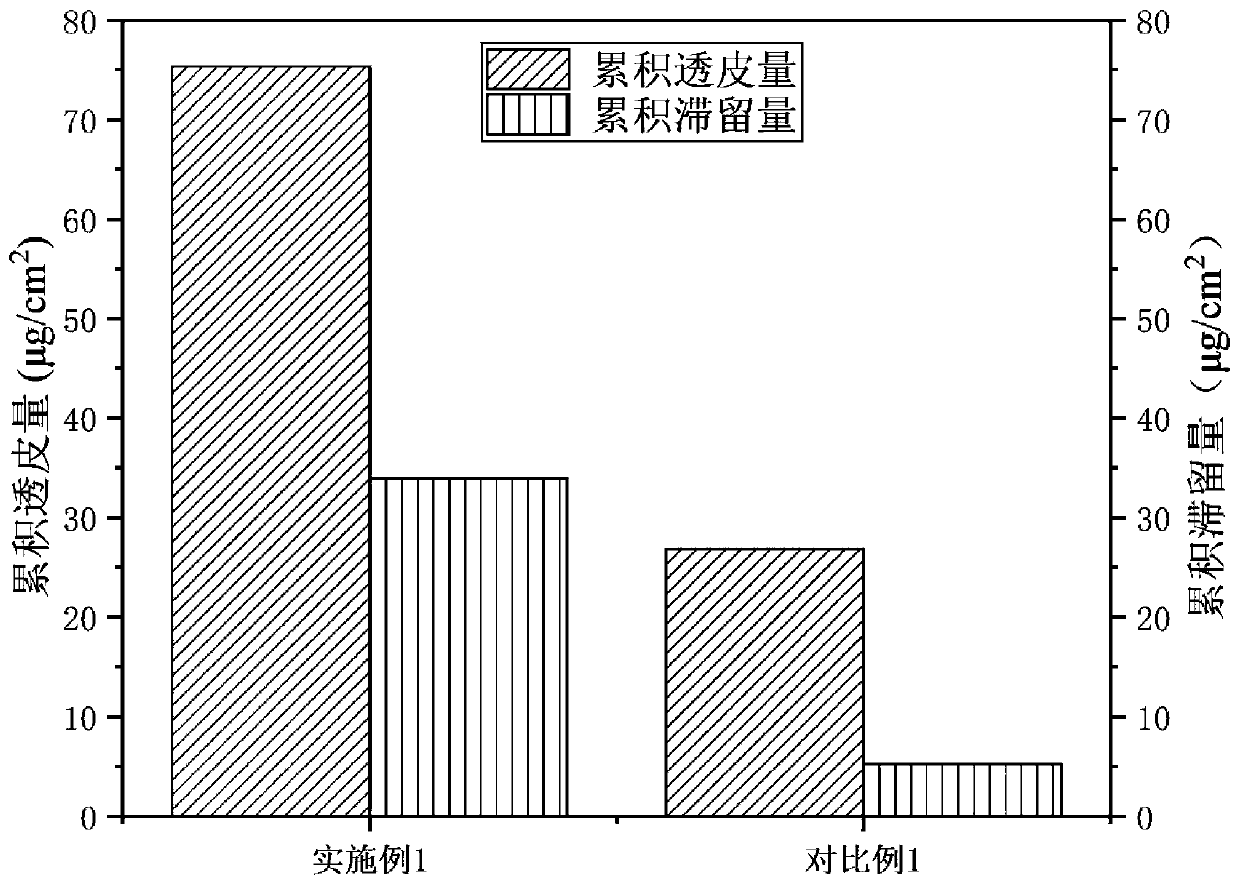
[0081] The transdermal properties of the samples were evaluated by vertical Franz diffusion cell method. Fix the isolated skin of SD rat abdomen between the receiving chamber and the supplying chamber of the diffusion cell, take 1g sample on the skin surface of the supplying chamber, the effective diffusion area is 3.14cm 2 , add physiological saline to the receiving tank as the receiving solution, drain the air bubbles so that the side of the dermis is in complete contact with the receiving solution, stir and diffuse at 32°C and 300r / min. Take 0.5mL of receiving solution at 4h, 8h, 12h, 16h, 20h, and 24h respectively, and add an equal amount of constant temperature blank receiving solution in time. The concentration of the polypeptide in the receiving solution was measured by HPLC, and the cumulative transdermal amount of the polypeptide per unit area at different times was calculated according to the following formula:
[0082]
[0083] Among th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com