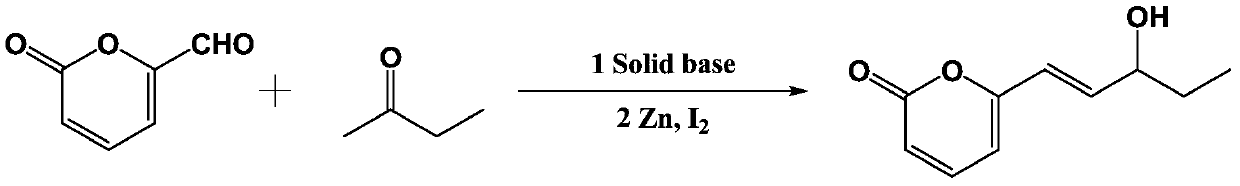Compound for treatment of psoriasis and preparation method of compound for treatment of psoriasis
A psoriasis and composition technology, applied in chemical instruments and methods, chemical/physical processes, medical preparations containing active ingredients, etc., can solve problems such as low yield, difficulty in large-scale production, and cumbersome steps. Achieve the effect of high product yield, large-scale production and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-
[0043] Example 1 - Preparation Example:
[0044]
[0045] Step 1: Preparation of solid base catalyst
[0046] Dissolve 2.0 g of sodium hydroxide in deionized water, impregnate 40.0 g of γ-alumina in equal volume, impregnate for 24 hours, then dry at 130°C for 15 hours, and then bake at 600°C for 4 hours to obtain sodium hydroxide supported on Solid base catalyst NaOH / Al on alumina 2 o 3 .
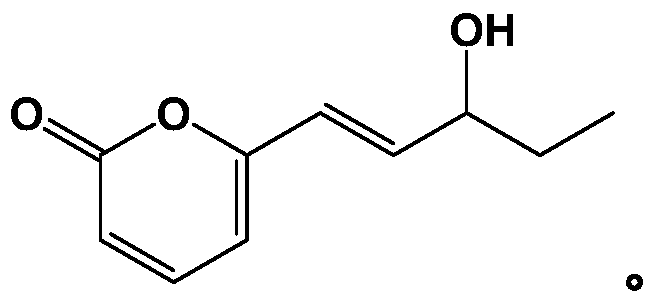
[0047] Step 2: Preparation of 6-(3-hydroxypent-1-enyl)-2H-pyran-2-one
[0048] Methyl ethyl ketone (5ml), the catalyst (8.4g) and ethanol (60ml) prepared in step 1 were mixed, and then 2H-pyran-2-one (1.24g, 10mmol) was dissolved in ethanol (20ml) dropwise. ) in the solution, dripped in 10 minutes, and stirred at room temperature to react. The progress of the reaction was monitored by TLC. After the raw material point of 2H-pyran-2-one completely disappeared, the solution was heated to 45° C. to continue the reaction for 1-2 h, so as to make the reaction complete. After cooling, fi...
Embodiment 3
[0059] Embodiment 3-pharmacological activity test embodiment:
[0061] 6-(3-Hydroxypent-1-enyl)-2H-pyran-2-one was dissolved in dimethyl sulfoxide, and then diluted with PBS buffer to prepare concentrations of 1000 μg / ml and 100 μg / ml respectively , 10 μg / ml, 1 μg / ml, 0.1 μg / ml and 0.01 μg / ml solutions. The sample solution was added to a 96-well plate, and human immortalized epidermal (HaCat) cells in growth phase (pharmacology laboratory, Shanghai Institute of Pharmaceutical Industry) were placed in the 96-well plate, and cultured for 48 hours. Then the inhibitory activity was determined by the standard MTT method, in which four parallel samples were set up for each concentration, and each experiment was repeated three times, and the conclusion was drawn through the control of the blank group. The OD value of each well was detected by a microplate reader, and the detection wavelength was 570nm. The cell inhibition rate was calculated as follo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com