Method for leaching manganese from electrolytic manganese anode residues through sulfuric acid curing
A technology for anode slag and electrolytic manganese, which is applied in the field of manganese leaching by sulfuric acid aging of electrolytic manganese anode slag, can solve the problems of high manganese leaching cost and secondary pollution, and achieve the effects of improving the leaching rate, improving the utilization rate and widening the source.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A method for leaching manganese from electrolytic manganese anode slag with sulfuric acid, specifically including the following steps:
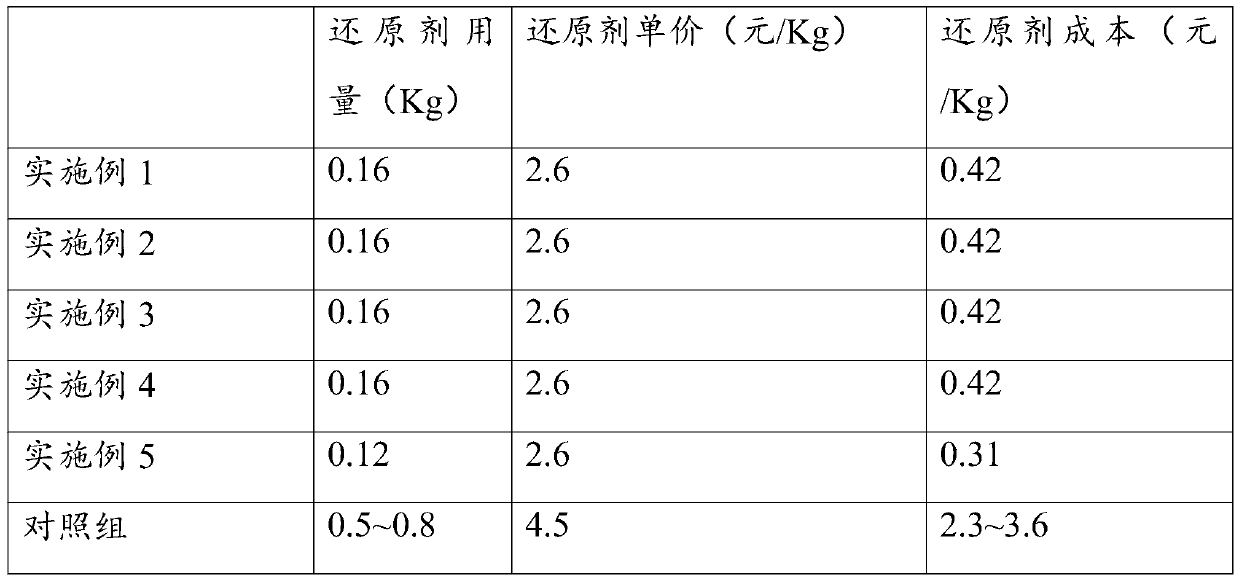
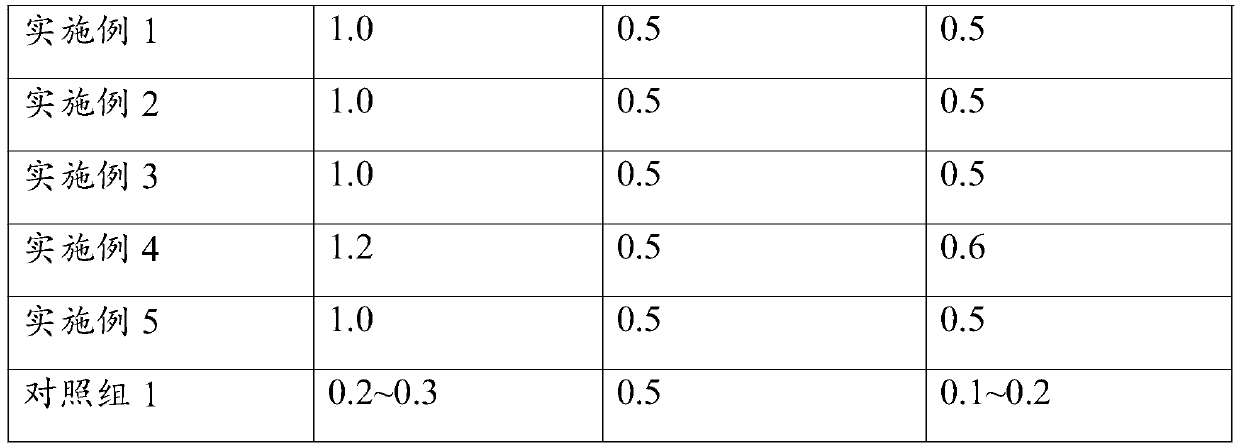
[0033] (1) Weigh 1Kg electrolytic manganese anode slag, 0.16Kg sulfur powder and 1Kg concentrated sulfuric acid (mass concentration> 95%);
[0034] (2) Add sulfur powder and concentrated sulfuric acid to the electrolytic manganese anode slag, stir until the mixture is uniform; heat to 125°C and mature for 20 hours to obtain clinker;
[0035] (3) Adding water to the clinker to adjust the slurry to a liquid-solid ratio of 2:1, stirring and leaching for 1 hour at room temperature, and filtering to obtain manganese-containing leaching solution and leaching residue. The leaching rate of manganese is 92.0%.
Embodiment 2
[0037] A method for leaching manganese from electrolytic manganese anode slag with sulfuric acid, specifically including the following steps:
[0038] (1) Weigh 1Kg electrolytic manganese anode slag, 0.16Kg sulfur powder and 1Kg concentrated sulfuric acid (mass concentration> 95%);
[0039] (2) Add sulfur powder and concentrated sulfuric acid to the electrolytic manganese anode slag, stir until the mixture is uniform; heat to 150°C and mature for 20 hours to obtain clinker;
[0040] (3) Adding water to the clinker to adjust the slurry to a liquid-solid ratio of 2:1, stirring and leaching for 1 hour at room temperature, and filtering to obtain manganese-containing leaching solution and leaching residue. The manganese leaching rate was 95.6%.
Embodiment 3
[0042] A method for leaching manganese from electrolytic manganese anode slag with sulfuric acid, specifically including the following steps:
[0043] (1) Weigh 1Kg electrolytic manganese anode slag, 0.16Kg sulfur powder and 1Kg concentrated sulfuric acid (mass concentration> 95%);
[0044] (2) Add sulfur powder and concentrated sulfuric acid to the electrolytic manganese anode slag, stir until the mixture is uniform; heat to 150°C and mature for 24 hours to obtain clinker;
[0045] (3) Adding water to the clinker to adjust the slurry to a liquid-solid ratio of 2:1, stirring and leaching for 1 hour at room temperature, and filtering to obtain manganese-containing leaching solution and leaching residue. The leaching rate of manganese is 97.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com