Synthesis method of nitrogen-containing heterocycles ferrocene derivative
A ferrocene derivative and a synthesis method technology are applied in the synthesis field of nitrogen-containing heterocyclic ferrocene derivatives, which can solve the problems of strict requirements for reagents and equipment, hazards, and difficulty in industrialized production and application, and achieve low production costs. , stable process, easy-to-obtain results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The embodiments of the present invention provide a synthetic method of nitrogen-containing heterocyclic ferrocene derivatives, the reaction scheme of the synthetic method includes:
[0032]
[0033] Wherein, R are independently selected from C1-C3 alkyl;
[0034] Described synthetic method comprises the steps:
[0035] (1) introducing an aldehyde group on the nitrogen-containing heterocycle in the chemical structure of the raw material a to obtain the intermediate b;
[0036] (II) Carrying out the hydroxylamination reaction of the aldehyde group in the chemical structure of the intermediate b to form a hydroxylamine group to obtain the intermediate c;
[0037] (III) Reducing the hydroxylamine group in the chemical structure of the intermediate c to obtain the nitrogen-containing heterocyclic ferrocene derivative 1.
[0038] In one specific embodiment, R is C1 alkyl (ie methyl). More specifically, the number of R is 4, all of which are C1 alkyl groups, and the abov...
Embodiment 1
[0056] A synthetic method of a nitrogen-containing heterocyclic ferrocene derivative in this embodiment, the reaction scheme is as follows:
[0057]
[0058] The steps of the synthetic method are as follows:
[0059] (I) Synthesis of intermediate b
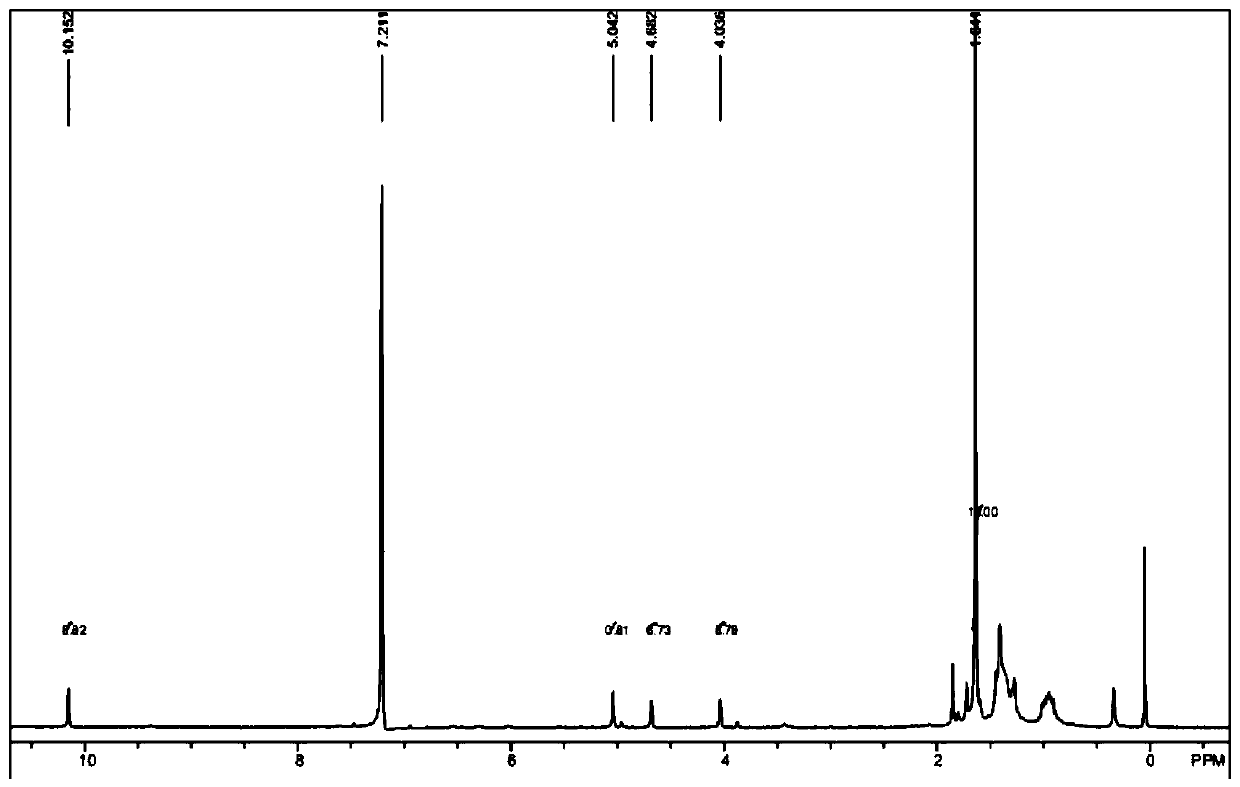
[0060] Add 400mg of raw material a (purchased from Aladdin, 1.5mmol), 7mL DMF (0.09mmol) into the reaction flask, lower the temperature of the system to 0°C, add 2mL of n-butyllithium solution (1.6M, 3.2mmol) dropwise, drop During the addition process, the temperature of the system is controlled at 0-5°C. After the dropwise addition, the temperature of the system is raised to 20-25°C to react for 1 hour; after the reaction is completed, add water to quench the reaction, extract with ethyl acetate, and distill the organic phase under reduced pressure to obtain intermediate Body b, dark red liquid 0.55g, purity 97%. 1H-NMR (300MHz, C 6 D. 6 )δ=10.15(s,1H), 5.04(s,1H), 4.68(s,1H), 4.04(s,1H), 1.64(s,15H), see the spectrum fig...
Embodiment 2
[0066] A synthetic method of a nitrogen-containing heterocyclic ferrocene derivative in this embodiment, its reaction scheme and steps are the same as in Example 1, the difference is that in step (1), n-butyllithium and N-methylmorpholine are used to introduce The formaldehyde.
[0067] Specifically, the steps of the synthesis method are as follows:
[0068] (I) Synthesis of intermediate b
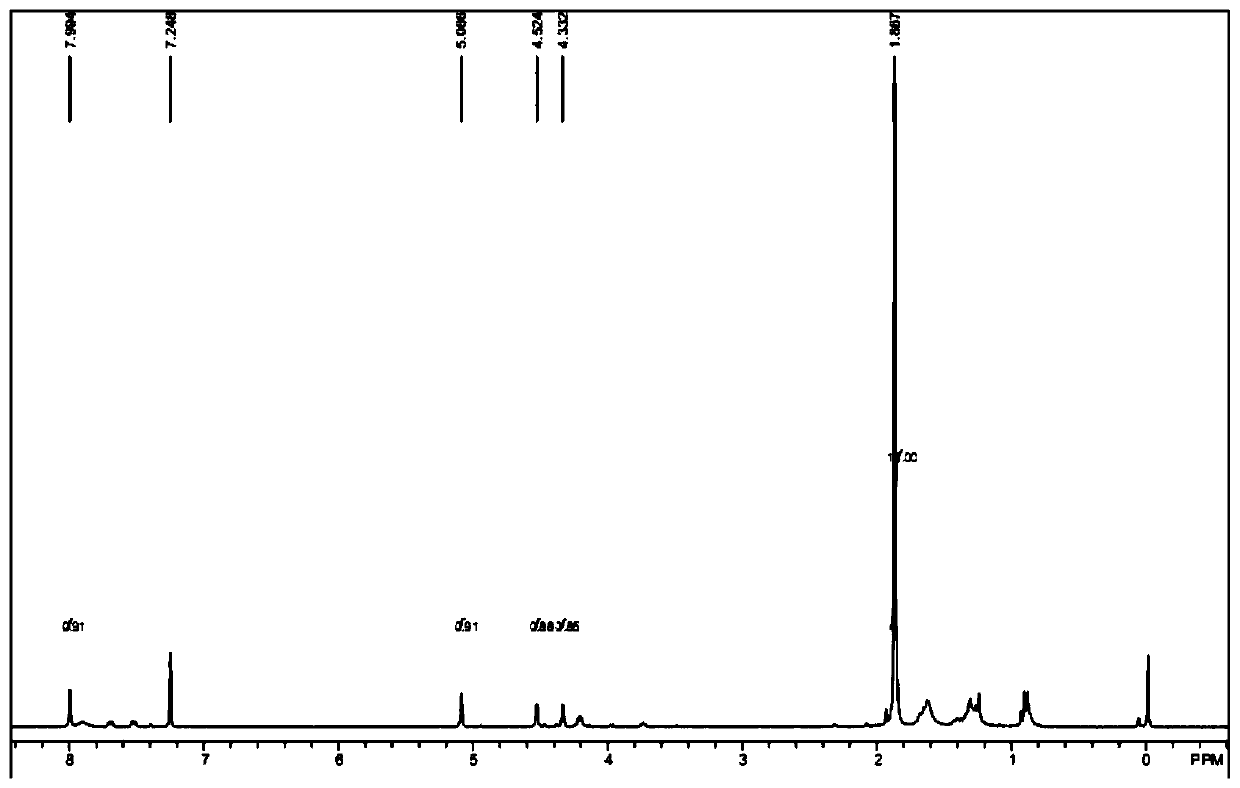
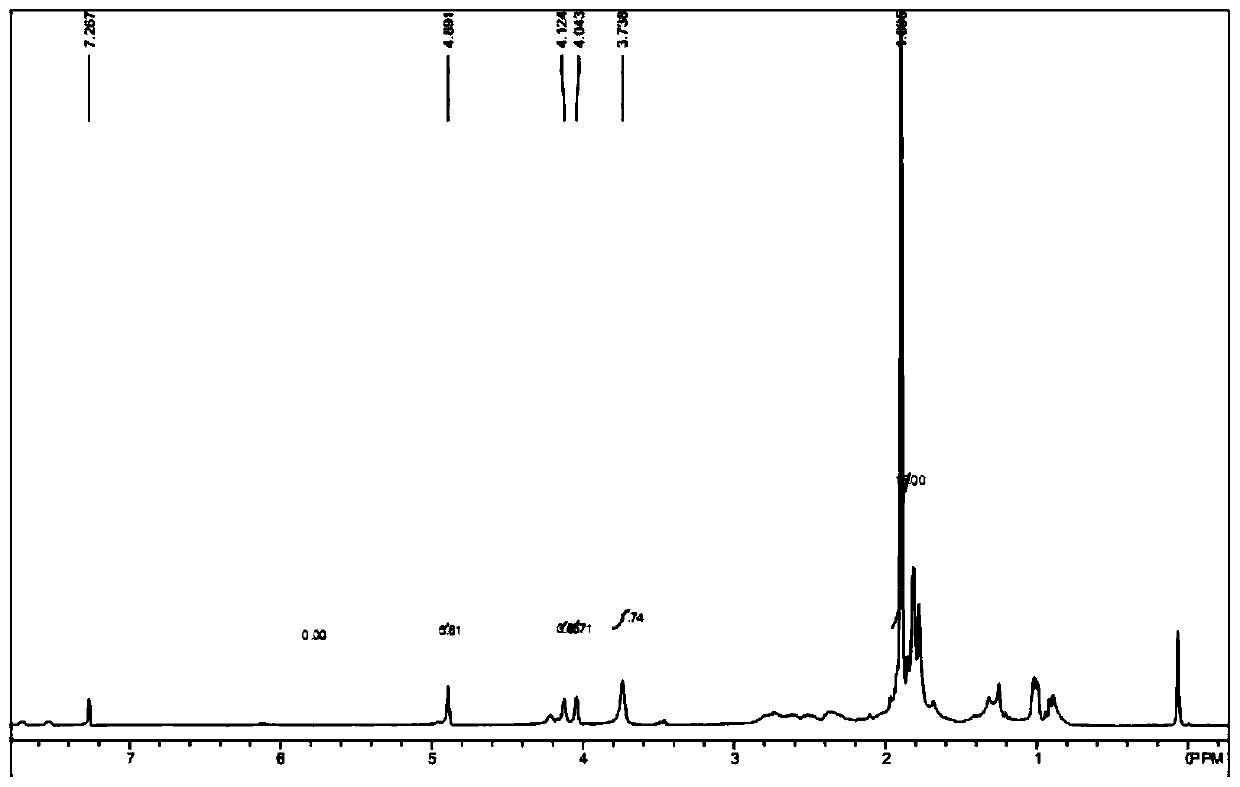
[0069] Add 400 mg of raw material a (purchased from Aladdin, 1.5 mmol), 7 mL of N-methylmorpholine (0.06 mmol) into the reaction flask, lower the temperature of the system to 0 ° C, and dropwise add 2 mL of n-butyllithium solution (1.6 M, 3.2mmol), the temperature of the system is controlled at 0-5°C during the dropwise addition, and after the dropwise addition, the system is heated to 20-25°C for 1 hour; after the reaction is completed, quench the reaction by adding water, extract with ethyl acetate, and subtract the organic phase Pressure distillation gave 0.5 g of dark red liquid with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com