SpyTag/SpyCatcher-cyclized L-beta-hydroxy-alpha-amino acid synthetase and application thereof
An amino acid and synthetase technology, applied in the direction of carbon-carbon lyase, enzyme, lyase, etc., can solve problems such as impact and unfavorable stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0019] Above-mentioned preparation method may comprise the steps:
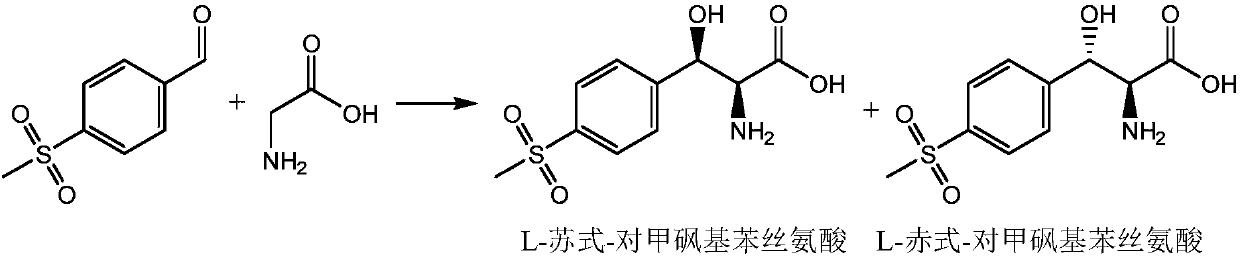
[0020] (a) reacting glycine and p-thymphenylbenzaldehyde in the presence of L-β-hydroxyl-α-amino acid synthetase cyclized with SpyTag / SpyCatcher in an aqueous solution system containing a cosolvent,
[0021] (b) solid-liquid separation to obtain a liquid phase comprising L-threo-p-thiamphenicol phenylserine and a solid phase comprising L-erythro-p-thiamphenicol phenylserine,
[0022] (c) cooling the liquid phase obtained in step (b) to precipitate L-threo-p-thiamphenicol phenylserine,
[0023] (d) solid-liquid separation to obtain precipitated L-threo-p-thiamphenicol phenylserine; and
[0024] (e) Optionally, the liquid phase resulting from step (d) is used in the reaction of step (a).
[0025] In the present invention, there is no special requirement on the amount of cyclized L-β-hydroxy-α-amino acid synthetase used in the reaction. If the dosage is small, the reaction will proceed slowly and the required ...
preparation example 1
[0050] Preparation of enzyme 24-1 having the amino acid sequence of SEQ No.1
[0051] (1) Synthesize the pET28a-24-1 plasmid, synthesize the DNA sequence SEQID No.3 gene capable of translating the amino acid sequence of SEQ ID No.1, and insert it between the BamHI-HindIII of the pET28a plasmid to obtain the recombinant plasmid pET28a -24-1, transforming the recombinant plasmid pET28a-24-1 into Escherichia coli E. coli BL21(DE3) to obtain the recombinant strain BL21(DE3) / pET28a-24-1.
[0052] (2) Cultivate the recombinant strain BL21(DE3) / pET28a-24-1 to express the enzyme 24-1, specifically comprising the following steps: the recombinant strain BL21(DE3) / pET28a-24-1 Bacterial colonies were inoculated into LB medium, shaken and cultured at 37°C for 12 hours, and the medium after shaking culture was transferred to lactose medium (peptone 10g / L, yeast powder 5g / L, Na 2 HPO4 12H 2 O 8.95g / L, KH 2 PO 4 3.4g / L, NH 4 Cl 2.67g / L, Na 2 SO 4 0.7g / L, MgSO 4 0.24g / L, glycerol 5...
preparation example 2
[0054] Preparation of enzyme KT2440 having the amino acid sequence of SEQ No.2
[0055] (1) Synthesize the pET28a-KT2440 plasmid, synthesize the DNA sequence SEQ ID No.4 gene capable of translating the amino acid sequence of SEQ ID No.2, and insert it between the BamHI-HindIII of the pET28a plasmid to obtain the recombinant plasmid pET28a-KT2440 , transforming the recombinant plasmid pET28a-KT2440 into Escherichia coli E. coli BL21(DE3) to obtain the recombinant strain BL21(DE3) / pET28a-KT2440.
[0056] (2) Cultivate the recombinant bacterial strain BL21(DE3) / pET28a-KT2440 to express the enzyme KT2440, specifically comprising the following steps: inoculating a single colony of the recombinant bacterial strain BL21(DE3) / pET28a-KT2440 into LB medium In the medium, cultured with shaking at 37°C for 12 hours, took the culture medium after shaking culture and transferred it to lactose medium (the same composition as in the enzyme preparation example 1) with an inoculum size of 2.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com