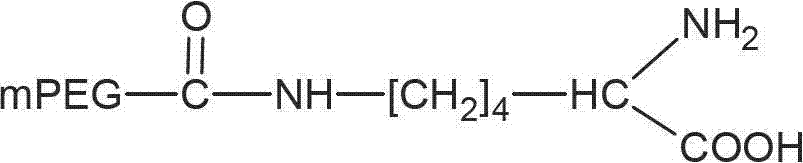Amino acid connected with polyethylene glycol and preparation method and application for amino acid
A polyethylene glycol and amino acid technology, applied in the direction of medical preparations of non-active ingredients, chemical instruments and methods, carrier binding/immobilizing peptides, etc., can solve the problems of not having side chain groups, etc., and achieve improved pharmacokinetics learning effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Preparation of mPEG-CO-ε-NH-Lys
[0045] Reaction formula:
[0046]
[0047] Take 1g monomethoxy polyethylene glycol acid 1000 (0.001mol), 0.74g α-tert-butoxycarbonyl-L-lysine (0.003mol) and 0.62g dicyclohexylcarbodiimide (DCC, 0.003mol ) was dissolved in 100ml of dichloromethane, stirred and refluxed at room temperature for 16 hours. The mixture was filtered, and the filtrate was evaporated to dryness under reduced pressure and dried in vacuo. The obtained solid matter was dissolved in 100ml of dichloromethane, and an appropriate amount of trifluoroacetic acid was added, stirred, then concentrated by rotary evaporation at 55°C and dried in vacuo. 100ml of cooled diethyl ether was added to the filtrate, and the resulting precipitate was filtered and dried in vacuo. The product mPEG-CO-ε-NH-Lys was 1.52g.
Embodiment 2
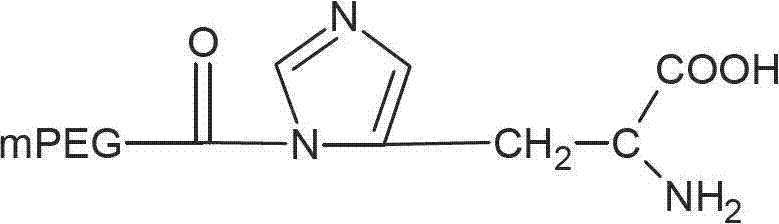
[0048] Embodiment 2: Preparation of mPEG-CO-His
[0049] Reaction formula:
[0050]
[0051] Dissolve 1 g of monomethoxypolyethylene glycol acid 1000 (0.001 mol) in 50 ml of dichloromethane, add thionyl chloride (2 ml, 0.004 mol) in dichloromethane, and stir overnight at room temperature. The mixture was rotary evaporated, and the resulting solid residue was dried in vacuo. The resulting solid matter was dissolved in 100 ml of dichloromethane, and 0.77 g of α-tert-butoxycarbonyl-L-histidine (0.003 mol) and 0.6 ml of triethylamine were added, and stirred overnight at room temperature. Filtrate, add an appropriate amount of trifluoroacetic acid to the filtrate and stir, then concentrate by rotary evaporation at 55°C and dry in vacuo. The obtained solid matter is dissolved in 50ml of dichloromethane and dried with anhydrous magnesium sulfate. After filtering, add 100ml of cooling diethyl ether to the filtrate. The resulting precipitate was filtered and dried in vacuo. The p...
Embodiment 3
[0052] Embodiment 3: Preparation of mPEG-CO-Trp
[0053] Reaction formula:
[0054]
[0055]Take 4g of monomethoxy polyethylene glycol acid 4000 (0.001mol) and dissolve it in 50ml of dichloromethane, add thionyl chloride (2ml, 0.004mol) in dichloromethane solution, and stir overnight at room temperature. The mixture was rotary evaporated, and the resulting solid residue was dried in vacuo. The obtained solid matter was dissolved in 100 ml of dichloromethane, 0.91 g of α-tert-butoxycarbonyl-L-tryptophan (0.003 mol) and 0.6 ml of triethylamine were added, and stirred overnight at room temperature. Filtrate, add an appropriate amount of trifluoroacetic acid to the filtrate and stir, then concentrate by rotary evaporation at 55°C and dry in vacuo. The obtained solid matter is dissolved in 50ml of dichloromethane and dried with anhydrous magnesium sulfate. After filtering, add 100ml of cooling diethyl ether to the filtrate. The resulting precipitate was filtered and dried in v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com