Regeneration method of cartilage.
A technology of cartilage and chondrocytes, applied in the field of regenerative medicine, can solve problems such as transient, tumorigenic, and short-term effects of growth factors, and achieve the effect of enhancing ability, reducing side effects, and lasting effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
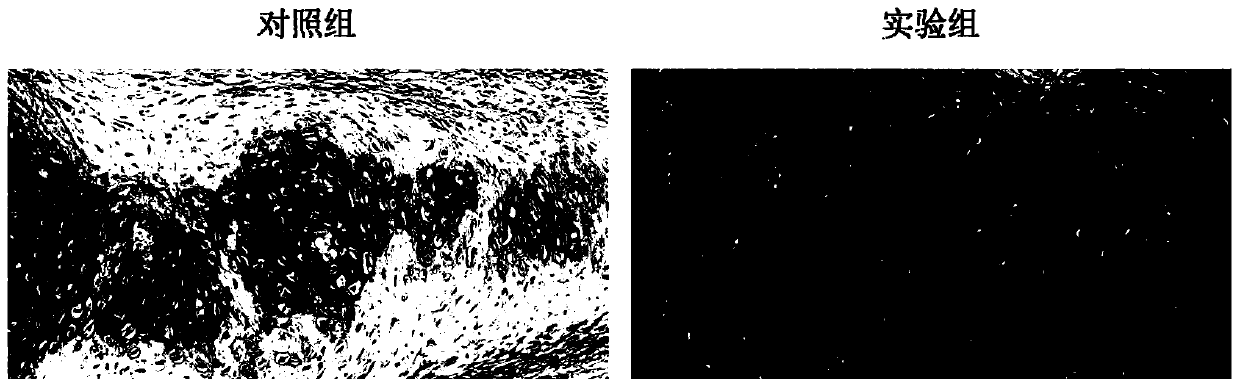
Image
Examples
Embodiment 1
[0029] This embodiment provides a cartilage regeneration method, which is a cartilage regeneration method based on gene transfection. The regeneration method includes the step of transfecting the target gene hsa-miR-193b-3p into chondrocytes.
[0030] In the step of transfecting the target gene hsa-miR-193b-3p into the chondrocytes, the target gene is transfected into the chondrocytes by the lentiviral vector, and at 35-39° C. and 4-6% CO 2 Incubate for 48-72 hours under the incubator conditions.
[0031] The regeneration method also includes: the step of culturing the transfected chondrocytes in vitro. In the step of culturing the transfected chondrocytes in vitro, the chondrocytes after successful transfection are routinely cultured in vitro to form a perichondrium sheet, that is, to obtain a cartilage regeneration material.
[0032] The acquisition of the chondrocytes comprises the following steps:
[0033] Take the residual ear cartilage discarded during the operation, c...
Embodiment 2
[0036] This embodiment provides a cartilage regeneration method, which includes the step of transfecting the target gene hsa-miR-193b-3p into chondrocytes.
[0037] In the step of transfecting the target gene hsa-miR-193b-3p into chondrocytes, the target gene is transfected into human chondrocytes by a lentiviral vector, and at 37° C. and 5% CO 2 Incubate for 60 hours in an incubator.
[0038] The regeneration method also includes: the step of culturing the transfected chondrocytes in vitro.
[0039] In the step of culturing the transfected chondrocytes in vitro, the chondrocytes after successful transfection are routinely cultured in vitro for 4 weeks to form a perichondrium sheet, that is, to obtain cartilage regeneration material.
[0040] The target gene contains GFP fluorescence, which is used to detect whether the target gene is successfully transfected into the chondrocytes.
[0041] The acquisition of the chondrocytes comprises the following steps:
[0042] Take the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com