Method for large-scale high-density suspension culture of cell 293-high yielding adenovirus
A suspension culture and high-density technology, applied in the field of biomedicine, can solve the problems of low toxin production, low cell density and yield, and achieve the effects of reducing cell culture loss, simplifying operations, and improving substances required for metabolism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
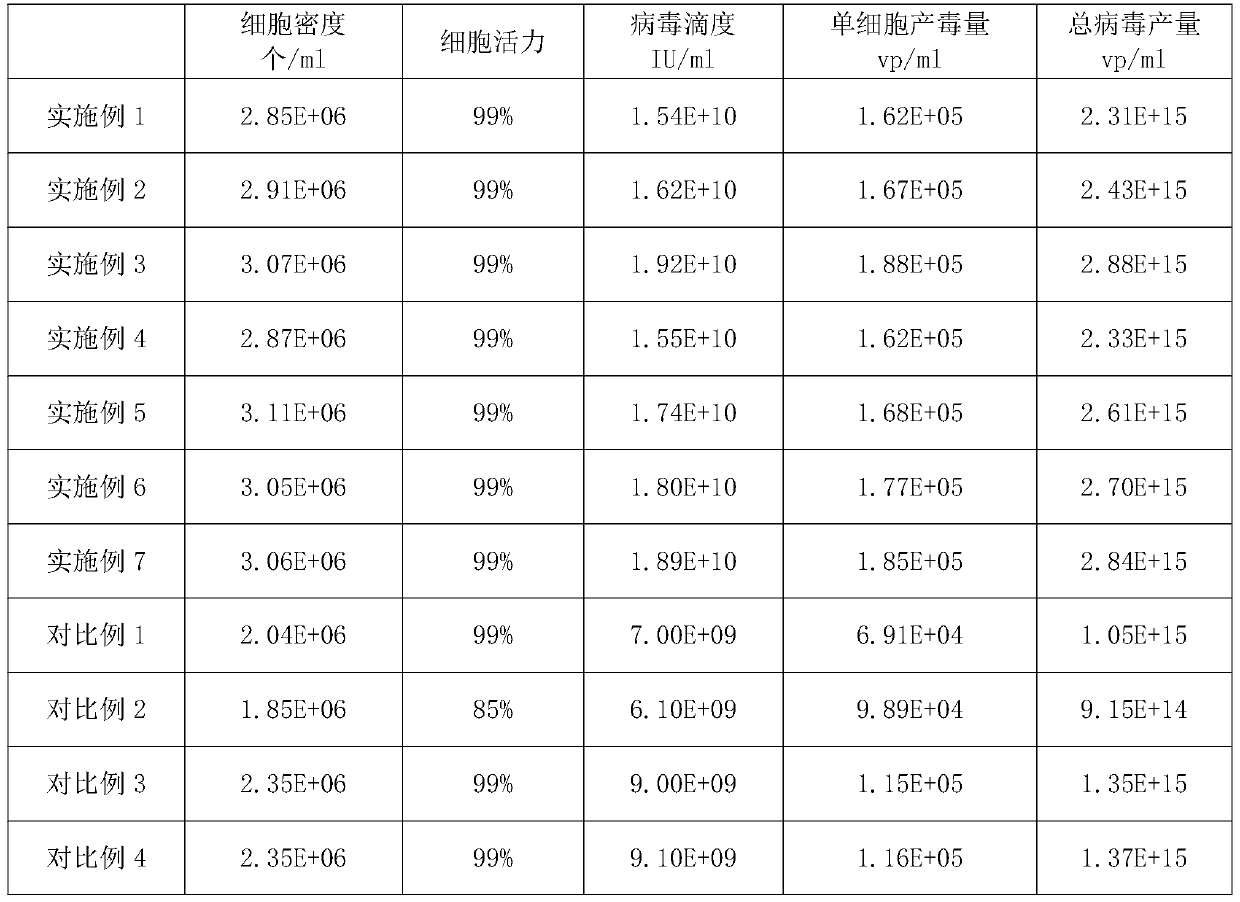
Examples
Embodiment 1
[0027] 1. The 293 parietal cells were taken and affixed to the serum-free 293SFM+serum gradient descending culture method, and finally became serum-free suspension cells.
[0028] 2. Suspension culture with serum-free 293SFM medium, first cultured to a certain concentration in a shaker flask, then transferred to a tank for cultivation, inoculated into a bioreactor at a certain cell density to adjust the culture conditions: culture temperature at 36 ° C; pH Maintained at 7.0; dissolved oxygen concentration at 30%; stirring speed at 80rpm, the stirring speed was set to 80rpm on the first day of cultivation, and the stirring speed was gradually increased at 120rpm from the second day of cultivation to continue culturing until the virus was infected.
[0029] 3. At the 45th hour of culture in the tank, perfusion was added to increase fresh serum-free 293SFM, and the required culture speed was 3L / d. At the same time, the cells in the excreted liquid were intercepted in the tank with...
Embodiment 2
[0032] 1. The 293 parietal cells were taken and affixed to the serum-free 293SFM+serum gradient descending culture method, and finally became serum-free suspension cells.
[0033] 2. Suspension culture with serum-free 293SFM medium, first cultured to a certain concentration in a shake flask, then transferred to a tank for cultivation, inoculated to a bioreactor at a certain cell density to adjust the culture conditions: culture temperature at 38 ° C; pH Maintained at 7.4; dissolved oxygen concentration at 50%; stirring speed at 160rpm, the stirring speed was set to 100rpm on the first day of cultivation, and the stirring speed was gradually increased at 160rpm from the second day of cultivation to continue culturing until the virus was infected.
[0034] 3. On the 52nd hour of culture in the tank, start perfusion to increase fresh serum-free 293SFM, and the required culture speed is 8L / d. At the same time, use a cell interceptor to trap the cells in the excreted liquid in the t...
Embodiment 3
[0037] 1. The 293 parietal cells were taken and affixed to the serum-free 293SFM+serum gradient descending culture method, and finally became serum-free suspension cells.
[0038] 2. Suspension culture with serum-free 293SFM medium, first cultured to a certain concentration in a shaker flask, then transferred to a tank for cultivation, inoculated to a bioreactor at a certain cell density to adjust the culture conditions: culture temperature at 37 ° C; pH Maintained at 7.2; dissolved oxygen concentration at 40%; stirring speed at 120rpm, the stirring speed was set to 90rpm on the first day of cultivation, and gradually increased at 140rpm from the second day of cultivation to continue culturing until virus infection.
[0039] 3. At the 48th hour of culture in the tank, start perfusion to increase fresh serum-free 293SFM, and the required culture speed is 5L / d. At the same time, use a cell interceptor to trap the cells in the excreted liquid in the tank, and start to infect the v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com