Vaccine based on fusion expression of ferritin and preS1 antigen gene
A ferritin and vaccine technology, applied in the direction of fusion polypeptide, virus antigen components, hybrid peptide, etc., can solve problems that need to be improved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1, construction and production of preS1-Ferritin
[0048] Materials: 1. Strains: Competent cells of DH5αE.coli and BL21(DE3)pLysS E.coli were purchased from Beijing Quanshijin Biotechnology Co., Ltd. 2. Plasmid: pDEST14,. 3. Gene fragments: the Ferritin gene was synthesized by Suzhou Jinweizhi Biotechnology Co., Ltd., and the preS1 gene was synthesized by Suzhou Jinweizhi Biotechnology Co., Ltd. according to the GenBank reference sequence KX470733.1.
[0049] method:
[0050] 1. Construction of pDEST14-preS1-Ferritin expression vector. Primers with restriction sites at both ends were designed respectively, and amplified by PCR. The nucleic acid sequences of the amplification primers for the preS1 peptide coding sequence are SEQ ID NO: 5 and SEQ ID NO: 6. The nucleic acid sequences of the amplification primers for the ferritin coding sequence are SEQ ID NO: 7 and SEQ ID NO: 8. The amplified preS1 peptide coding sequence and ferritin coding sequence and th...
Embodiment 2
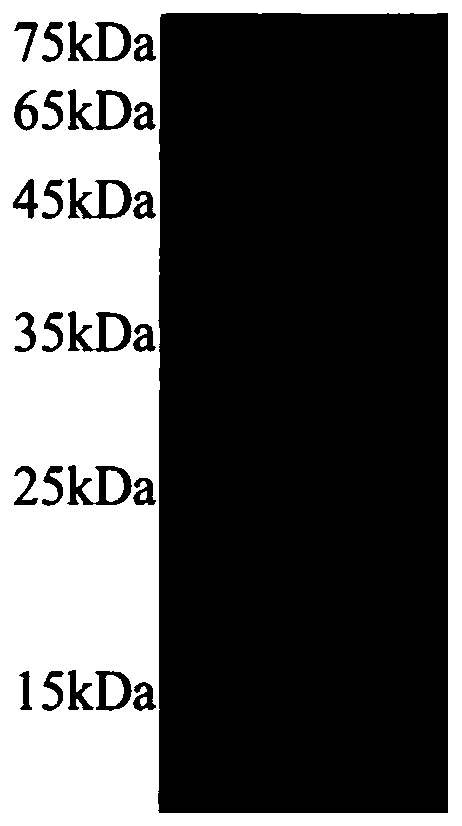
[0054] Example 2, Purification and Characterization of pDEST14-preS1-Ferritin
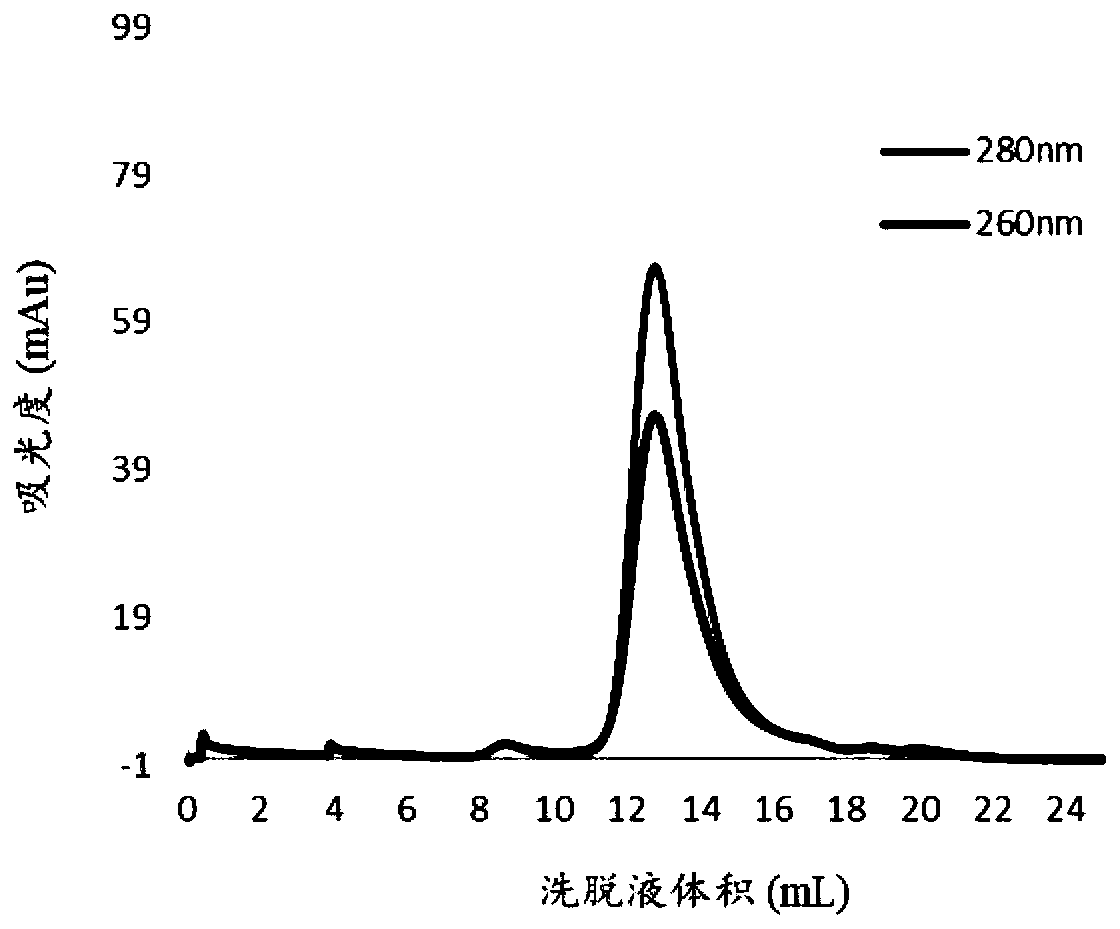
[0055] 1. Crude and pure. The induced bacteria were resuspended in the buffer solution, namely 20mM Tris-HCl, 50mM NaCl, PH=7.5, sonicated with 30% power of the sonicator, cooled for 5s every 5s, sonicated for 15min in total, about 90 cycles . Centrifuge at 12,000 rpm for 15 minutes to discard the supernatant, and then resuspend the pellet with buffer 2, namely 20 mM Tris-HCl, 50 mM NaCl, pH=9.5. After sufficient resuspension, centrifuge again at 12000rpm for 15min, and collect the supernatant. Filter with a 0.22 μm filter to remove impurities such as precipitates. At this time, most of the protein in the supernatant is the target protein.
[0056] 2. Gel filtration column purification. Equilibrate the Superose6Increase gel filtration column with 20mM Tris-HCl, 50mM NaCl, pH=9.5 buffer solution, add 0.5ml of crude protein solution after passing through a 0.22μm filter membrane, and continue to ...
Embodiment 3
[0059] Antibody response detection caused by vaccines such as embodiment 3, preS1-Ferritin
[0060] Materials: Horseradish peroxidase-labeled goat anti-mouse IgG was purchased from Zhongshan Jinqiao Company. The TLR9 ligand mouse CpG-B adjuvant (ODN1826, 5′-tccatgacgttcctgacgtt-3′, all nucleotides are sulfur-modified) was synthesized by Shanghai Jierui Bioengineering Co., Ltd. C57BL / 6 female mice (6-8 weeks) were purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd.
[0061] method:
[0062] 1. PreS1-Ferritin and other nano-vaccine immunization mice. 500pmol (calculated by monomer) of preS1-Ferritin vaccine or 500pmol SC-preS1 control vaccine one or 500pmol preS1-SC-ST-Ferritin control vaccine two were subcutaneously immunized 8-week-old C57BL / 6 mice with 30 μg (according to order Body calculation) TLR9 ligand CpG as adjuvant, diluted with PBS to 100 μl of immune system per mouse. Wherein, the SC-preS1 control vaccine refers to the vaccine comprisin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com