Green reactive dye based on mono-H acid triazo structure as well as preparation and application of green reactive dye
A technology of reactive dyes and trisazo, which is applied in the field of green reactive dyes and its preparation, can solve problems such as insufficient lifting performance and color fastness, and poor compatibility, and achieve novel shades, good strength performance and color fastness, and avoid The effect of poor compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The synthetic technique of the green reactive dye with following structure:
[0048]
[0049] (1) Preparation of diazonium salt of anthranilsulfonic acid: Accurately weigh 17.3g (0.10mol) of anthranilsulfonic acid, configure it into an aqueous solution with a mass fraction of 20%, stir for 0.5h until the mixture is uniform, then cool down to 0~ 5°C, add 10.0g (0.10mol) of concentrated hydrochloric acid with a mass fraction of 37.5%, and stir for 10 minutes, then slowly add 6.969g (0.101mol) of sodium nitrite dropwise to prepare a 30% aqueous solution. React at 5°C for 2 hours, and use sulfamic acid to eliminate excess nitrous acid after the reaction.
[0050] (2) Coupling reaction: Accurately weigh 15.1g m-ureidoaniline, configure it into an aqueous solution with a mass fraction of 20%, add 1.7g NaHCO 3 Dry powder, stirred for 0.5h, and cooled to 0 ~ 5 ° C, the diazonium salt in step (1) was poured into the m-ureidoaniline solution, and the mass fraction was 15% Na ...
Embodiment 2
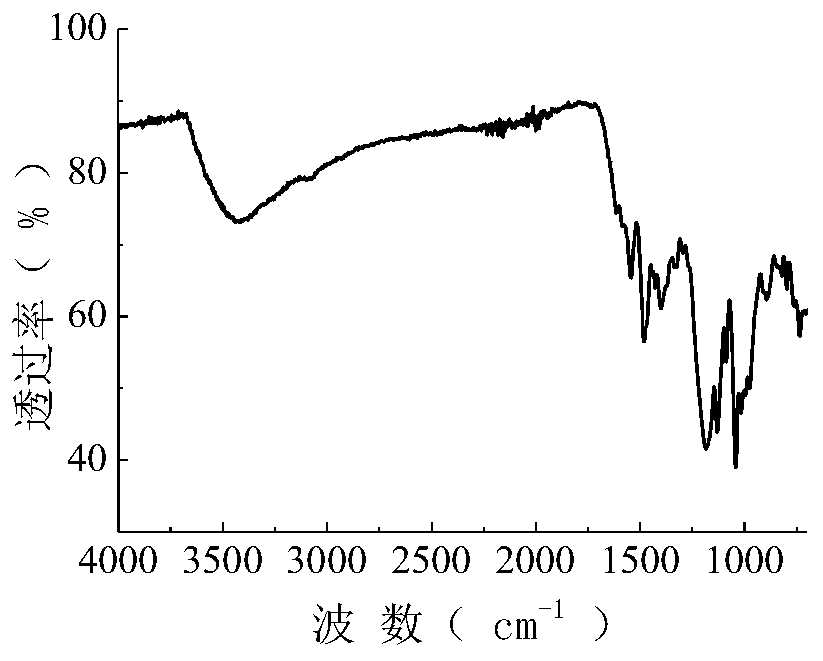
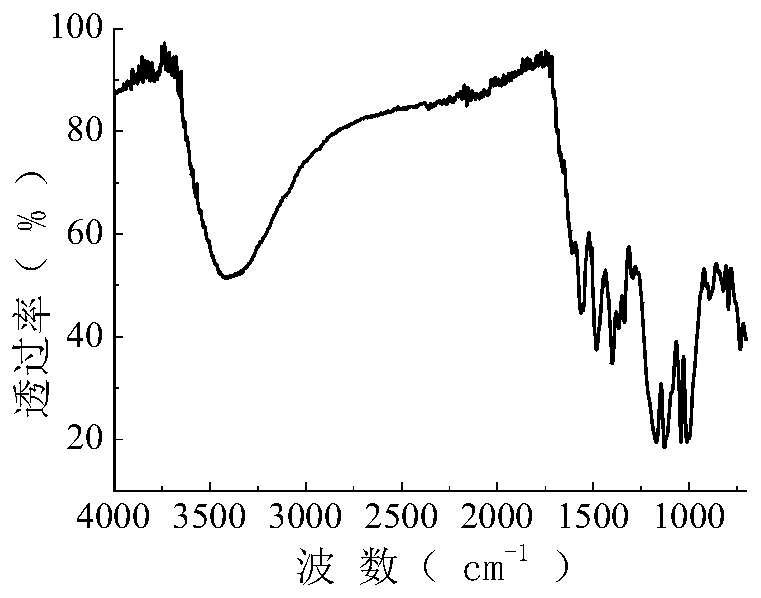
[0058] The synthetic route of the reactive dye of this embodiment is the same as that of Example 1, and the raw material 17.3g (0.10mol) anthranilic acid is changed into 27.5g (0.10mol) aniline-2,5-disulfonic acid in Example 1, and all the other are Same as Example 1, a green reactive dye with the following structural formula was obtained with a yield of 74.9% (yield calculated by aniline-2,5-bissulfonic acid). After the dye is purified by multiple recrystallizations from a mixture of ethanol and water (2:1), its infrared spectrum can be seen in figure 2 , the data are as follows: 3417.3, 1563.3, 1483.9, 1400.9, 1366.5, 1337.6, 1175.8, 1171.0, 1129.4, 1042.7, 1012.4, 894.6, 821.9, 796.0cm -1 .
[0059]
Embodiment 3
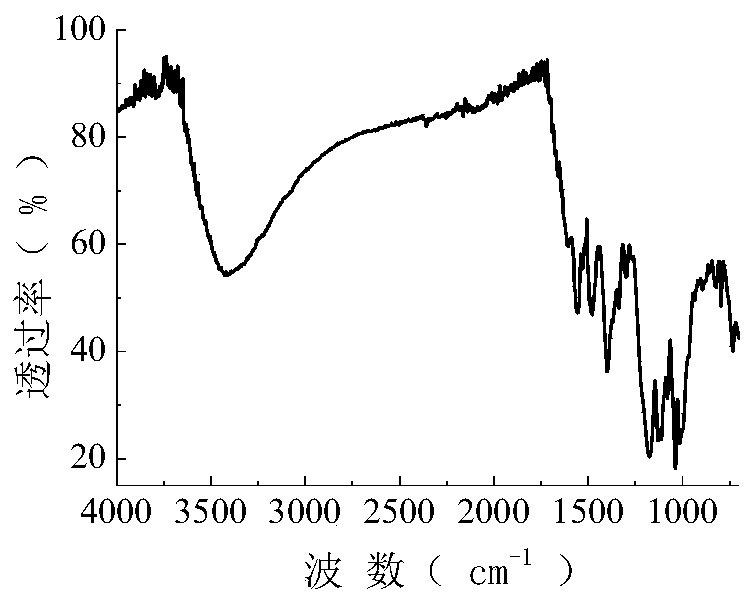
[0061] The synthetic route of the active dye of this example is the same as that of Example 1, and the raw material 17.3g (0.10mol) of anthranilic acid in Example 1 is replaced with 38.3g (0.10mol) of 2-naphthylamine-3,6,8-tri Sulfonic acid, all the other are identical with embodiment 1, obtain the green reactive dye of structural formula following, productive rate is 76.5% (calculate productive rate with 2-naphthylamine-3,6,8-trisulfonic acid). After the dye is purified by multiple recrystallizations from a mixture of ethanol and water (2:1), its infrared spectrum can be seen in image 3 , the data are as follows: 3426.8, 1556.1, 1530.2, 1479.2, 1400.8, 1337.2, 1299.9, 1174.2, 1129.8, 1081.1, 1038.7, 1015.2, 822.6cm -1 .
[0062]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com