Industrial preparation method of anti-asthma drug pranlukast intermediate
An intermediate and industrial technology, which is applied in the field of medical chemical drugs, can solve the problems of low total yield, low purity, and poor color of pranlukast, and achieve the effect of good color, high purity, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
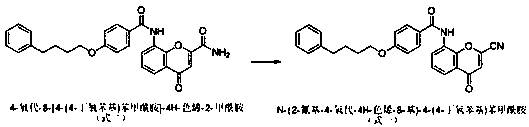
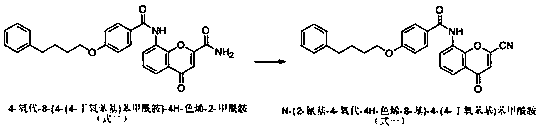
[0020] Example 1 Preparation of Pranlukast intermediate N-(2-cyano-4-oxo-4H-chromen-8-yl)-4-(4-butoxyphenyl)benzamide
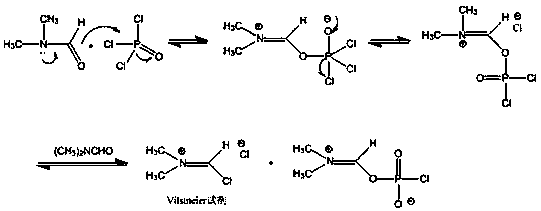
[0021] Purification of phosphorus oxychloride: 100 kg of commercially available industrial grade phosphorus oxychloride is added to the distillation tower, and 20 kg of the former fraction is evaporated under normal pressure, leaving 80 kg of phosphorus oxychloride for use.
[0022] Add 400kg of DMF to the dry reaction kettle, cool to 0°C, add 67kg (4 equivalents) of purified phosphorus oxychloride dropwise, control the temperature within 10°C, keep stirring for 30min after dropping, add 4-oxo-8 -[4-(4-butoxyphenyl)benzamide]-4H-chromene-2-carboxamide 50kg, continue to keep warm for 4 hours. Add 1500 kg of ice-brine to another reaction kettle, slowly transfer the reaction solution into ice-brine, the temperature does not exceed 10°C, stir for 30 minutes, press filter, wash the filter cake to neutral, and dry to obtain N-(2-cyanide Base-4-oxo-4H-chromen-8-yl)...
Embodiment 2
[0023] The preparation of embodiment 2 pranlukast intermediate N-(2-cyano-4-oxo-4H-chromen-8-yl)-4-(4-butoxyphenyl)benzamide
[0024] Purification of phosphorus oxychloride: 100 kg of commercially available industrial grade phosphorus oxychloride is added to the distillation tower, and 10 kg of the former fraction is evaporated under normal pressure, leaving 90 kg of phosphorus oxychloride for use.
[0025] Add 400kg of DMF to the dry reaction kettle, cool to 0°C, add 84kg (5 equivalents) of purified phosphorus oxychloride dropwise, control the temperature within 10°C, keep stirring for 30min after dropping, add 4-oxo-8 -[4-(4-butoxyphenyl)benzamide]-4H-chromene-2-carboxamide 50kg, continue to keep warm for 4 hours. Add 1500 kg of ice-brine to another reaction kettle, slowly transfer the reaction solution into ice-brine, the temperature does not exceed 10°C, stir for 30 minutes, press filter, wash the filter cake to neutral, and dry to obtain N-(2-cyanide Base-4-oxo-4H-chrome...
Embodiment 3
[0026] Embodiment 3 Preparation of pranlukast intermediate N-(2-cyano-4-oxo-4H-chromen-8-yl)-4-(4-butoxyphenyl)benzamide
[0027] Purification of phosphorus oxychloride: 100 kg of commercially available industrial grade phosphorus oxychloride is added to the distillation tower, and 20 kg of the former fraction is evaporated under normal pressure, leaving 80 kg of phosphorus oxychloride for use.
[0028] Add 400kg of DMF to the dry reaction kettle, cool to 0°C, add 33.5kg (2 equivalents) of purified phosphorus oxychloride dropwise, control the temperature within 10°C, keep stirring for 30min after dropping, add 4-oxo- 50kg of 8-[4-(4-butoxyphenyl)benzamide]-4H-chromene-2-carboxamide was kept for 4 hours of heat preservation reaction. Add 1500 kg of ice-brine to another reaction kettle, slowly transfer the reaction solution into ice-brine, the temperature does not exceed 10°C, stir for 30 minutes, press filter, wash the filter cake to neutral, and dry to obtain N-(2-cyanide Bas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com