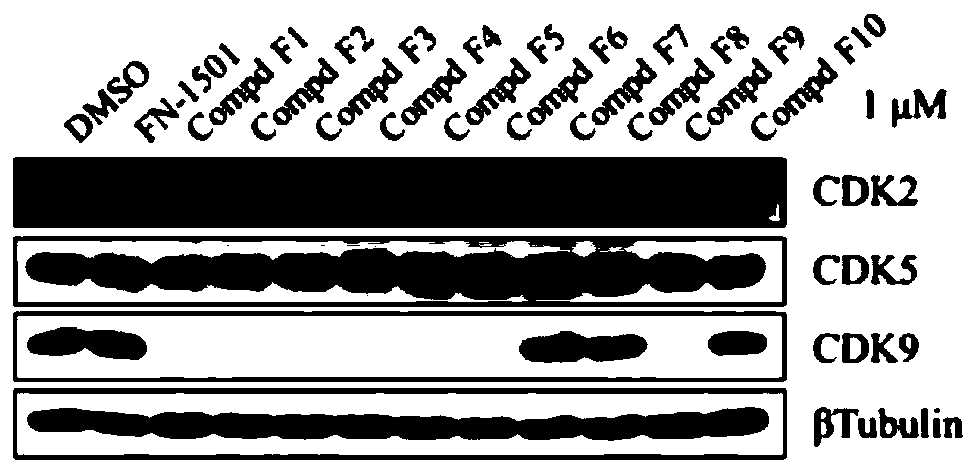
Compound for selectively degrading CDK subtype in targeted manner and application thereof
A compound and selective technology, applied in the field of biomedicine, can solve the problems of serious toxicity, poor treatment effect, hindering clinical development, etc., and achieve the effect of preventing proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
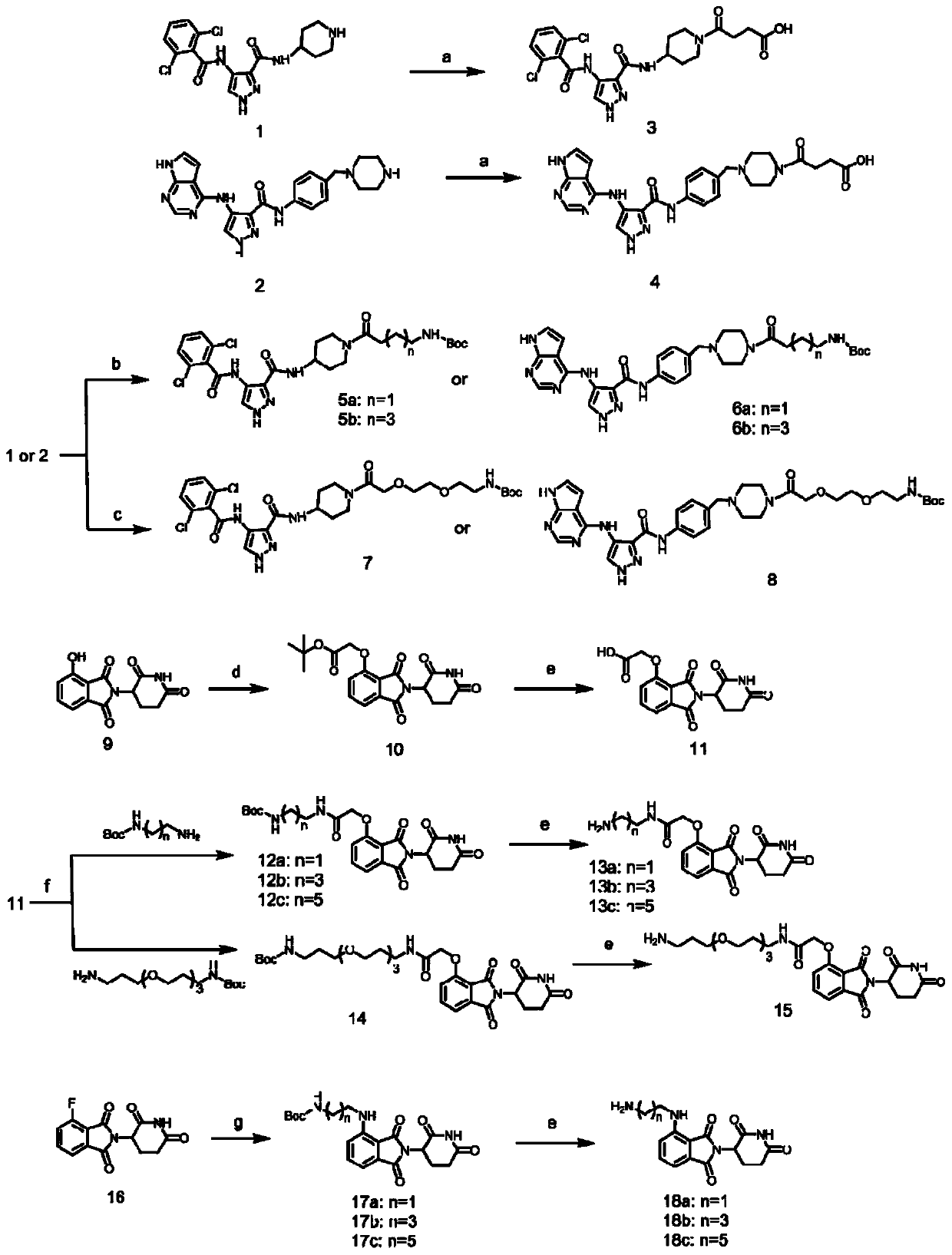
[0111] One, the preparation of each intermediate
[0112] 1) Intermediate 1 can be purchased directly, CAS No.: 844442-38-2;
[0113] 2) The synthetic route of intermediate 2 is as follows:
[0114]
[0115] The specific preparation method is: take 5mmol 4-(4-aminobenzyl) piperazine-1-tert-butyl carboxylate CAS No: 304897-49-2 and 7.5mmol HATU and dissolve it in 30mL DMF, then add 10mmol DIPEA and 5.5mmol 4-nitropyrazole-3-carboxylic acid CAS No: 5334-40-7, reacted at room temperature for 12h. The reaction was quenched with water, extracted three times with ethyl acetate, and then passed through a silica gel column with petroleum ether: ethyl acetate 3:1 to obtain compound A with a yield of 76%.
[0116] Weigh 2mmol of Compound A and dissolve in 10mL of ethanol, then add 6mmol of reduced iron powder and 3mmol of ammonium chloride, heat up to 80°C and reflux for 6h. Pad Celite suction filter the reaction solution, then spin the reaction solution to dryness, and use dichlo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com