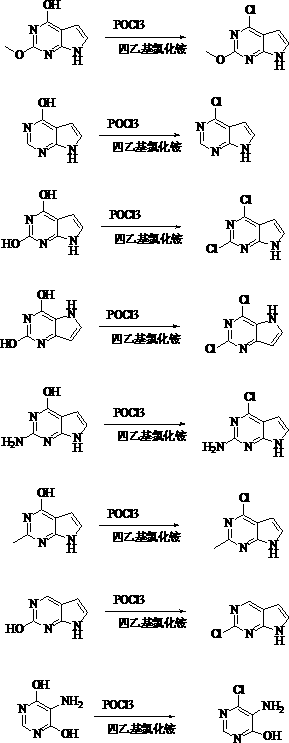
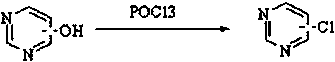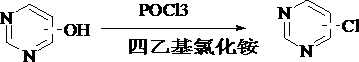Preparation method for catalyzing pyrimidine cyclic hydroxyl chlorination by tetraethylammonium chloride
A technology of tetraethylammonium chloride catalyzing the chlorination of pyrimidine ring hydroxy, tetraethylammonium chloride, applied in the direction of organic chemistry, etc., can solve the problems of high pressure of phosphorus oxychloride, large environmental pollution, low catalytic efficiency, etc. Achieve the effect of less environmental pollution, high catalytic efficiency and light color
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Preparation of 4-chloropyrrolopyrimidine
[0050] In a 2L three-neck flask equipped with a thermometer and a condenser, add 184g of phosphorus oxychloride and 16.6g of tetraethylammonium chloride, raise the temperature to 60°C, add 135g of 4-hydroxypyrrolopyrimidine in batches, raise the temperature to 80°C, and keep the temperature for reaction After 30 minutes, the reaction of the raw materials was traced by HPLC, and the temperature was lowered to 30°C.
[0051] Prepare 2 liters of 10% sodium hydroxide solution, slowly add the reaction solution dropwise to extract, and precipitate a solid, filter with suction, and dry to obtain 135 g of the finished product, with a yield of 87.6%.
[0052] 1H NMR (400 MHz, DMSO-ds) δ 12.58 (bs, 1H), 8.58 (s, 1H), 7.69 (d, 1H, J=3.5 Hz), 6.59 (d, 1H, J=3.5 Hz) ppm;
Embodiment 2
[0053] Example 2: Preparation of 2,4-dichloro-7H pyrrole [2,3-D] pyrimidine
[0054] In a 2L three-necked flask equipped with a thermometer and a condenser, add 306g of phosphorus oxychloride and 166g of tetraethylammonium chloride, raise the temperature to 100°C, and add 151g of 2,4-dihydroxy-7H pyrrole [2,3-D ] pyrimidine, the temperature was raised to 110°C, and the temperature was kept for 12 hours, and the reaction of the raw materials was traced by HPLC, and the temperature was lowered to 30°C.
[0055]Prepare 2 liters of 10% sodium hydroxide solution, slowly add the reaction solution dropwise to extract, and precipitate a solid, filter with suction, and dry to obtain 140 g of the finished product, with a yield of 74.5%.
[0056] 1H-NMR (400 MHz, DMSO-d6) ö 12.79 (s, 1H), 7.94 — 7.24 (m, 1H), 6.66(ddd, J= 5.3, 3.5, 1.7 Hz, 1H);
Embodiment 3
[0057] Example 3: Preparation of 2,4-dichloro-7H pyrrole [3,2-D] pyrimidine
[0058] In a 2L three-necked flask equipped with a thermometer and a condenser, add 306g of phosphorus oxychloride and 166g of tetraethylammonium chloride, raise the temperature to 100°C, and add 151g of 2,4-dihydroxy-7H pyrrole [3,2-D ] pyrimidine, the temperature was raised to 110°C, and the temperature was kept for 12 hours, and the reaction of the raw materials was traced by HPLC, and the temperature was lowered to 30°C.
[0059] Prepare 2 liters of 10% sodium hydroxide solution, slowly add the reaction liquid dropwise to extract, and precipitate a solid, filter with suction, and dry to obtain 151 g of the finished product, with a yield of 80.3%.
[0060] 1H NMR (400 MHz, DMSO-d6): δ 6.71 (d, 1H, J=3.2 Hz), 8.09 (d, 1H, J=2.8 Hz), 12.75 (s, 1H, NH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com