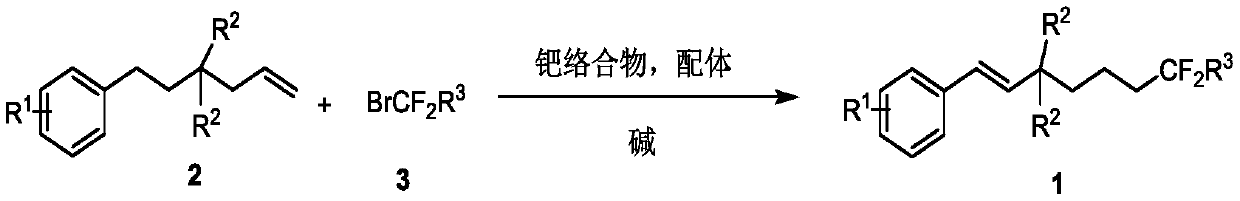
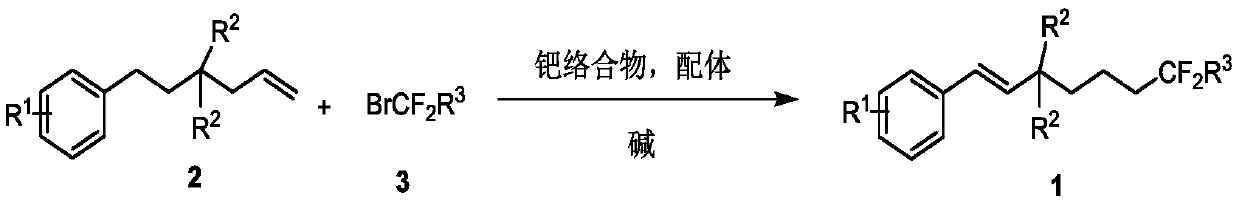
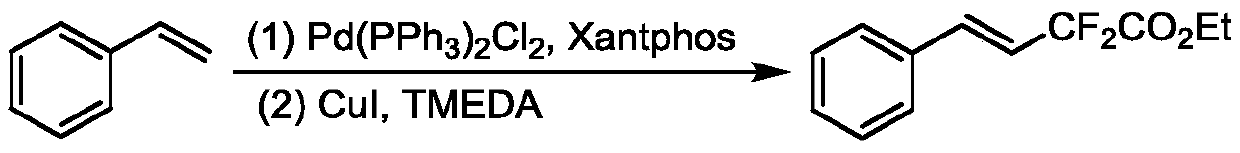Method for synthesizing remote fluorinated aryl olefin
A substituted aryl olefin, remote technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problem of not being applicable to the preparation of remote fluoro aryl olefins, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]
[0031] In the reaction flask, continuously bubble nitrogen into the reaction flask, then add 2-allyl-2-phenylethylmalonate 2a (30.4 mg, 0.1 mmol), ethyl bromodifluoroacetate 3a (40.2mg), bis(triphenylphosphine)palladium dichloride (3.5mg), 4,5-bisdiphenylphosphine-9,9-dimethylxanthene (5.78), potassium carbonate (27.6mg ) and 1.0 mL of acetonitrile, stirred and mixed evenly, reacted at 80 degrees for 12 hours, and separated directly by column chromatography to obtain the target product 1a with a yield of 72%. 1 H NMR (600MHz, CDCl 3 )δ7.41(d, J=7.4Hz, 2H), 7.32(t, J=7.4Hz, 2H), 7.26(t, J=7.5Hz, 1H), 6.74(d, J=16.6Hz, 1H) ,6.49(d,J=16.6Hz,1H),4.29-4.21(m,6H),2.20(t,J=8.5Hz,2H),2.13-2.04(m,2H),1.49-1.43(m,2H ),1.30-1.25(m,9H); 19 F NMR (564MHz, CDCl 3 )δ-105.86(t, J=16.9Hz, 2F); 13 C NMR (150MHz, CDCl 3 )δ170.18, 164.08(t, J=32.9Hz), 136.30, 131.56, 128.55, 127.99, 126.52, 125.73, 115.87(t, J=249.1Hz), 62.79, 61.70, 59.21, 34.95, 34.49(t, J=23.1 Hz), 16.64, 13...
Embodiment 2
[0034]
[0035] In the reaction flask, continuously bubble nitrogen into the reaction flask, then add 2-allyl-2-(4-methoxy) phenethyl malonate 2b (32.0mg, 0.1mmol), bromine Ethyl difluoroacetate 3a (40.2mg), bis(triphenylphosphine) palladium dichloride (3.5mg), 4,5-bisdiphenylphosphine-9,9-dimethylxanthene (5.78 ), potassium carbonate (27.6 mg) and 1.0 mL of acetonitrile, stirred and mixed evenly, reacted at 80 degrees for 12 hours, and separated directly by column chromatography to obtain the target product 1b with a yield of 78%. 1 HNMR (600MHz, CDCl 3)δ7.35(d, J=8.8Hz, 2H), 6.86(d, J=8.6Hz, 2H), 6.58(d, J=16.6Hz, 1H), 6.42(d, J=16.6Hz, 1H) ,4.29(q,J=7.3Hz,2H),4.24–4.20(m,4H),3.81(s,3H),2.18(t,J=8.5Hz,2H),2.12-2.04(m,2H), 1.47-1.42(m,2H),1.29(t,J=7.0Hz,3H),1.25(t,J=7.0Hz,6H); 19 F NMR (564MHz, CDCl 3 )δ-105.87(t, J=15.9Hz, 2F); 13 C NMR (150MHz, CDCl 3 )δ170.34, 164.10(t, J=32.9Hz), 159.48, 130.96, 129.11, 127.75, 123.36, 115.90(t, J=248.9Hz), 113.94, 62.79, 61.65, ...
Embodiment 3
[0037] Adopt the identical reaction conditions of embodiment 1-2, only change substrate 2, obtain serial product 1c-1o, concrete result is as follows: (wherein, Rf=CF 2 CO 2 Et)
[0038]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com