Preparation method of SPDIB
A dripping and filter cake technology, applied in the field of biomedicine, to achieve the effect of low raw material cost and short steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
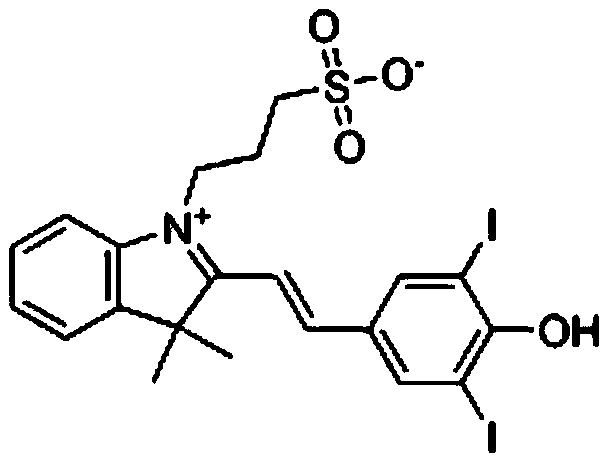
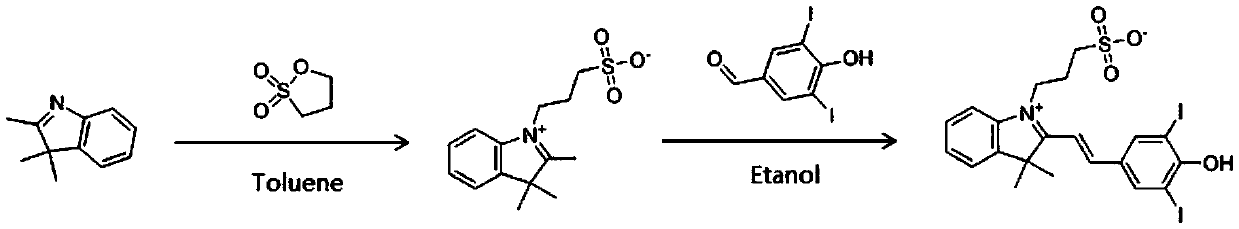
[0017] A kind of preparation method of SPDIB of the present invention, comprises the steps:
[0018] ① Dissolve 2,3,3-trimethyl-3H-indole in toluene, add 1,3-propane sultone dropwise, heat up to 120°C for reflux reaction for 4-5 hours after dropping, then cool to room temperature, Filter to obtain filter cake;
[0019] ② recrystallize the filter cake obtained in step ① to obtain light yellow powder;
[0020] ③Co-dissolve the light yellow powder obtained in step ② with 3,5-diiodo-4-hydroxybenzaldehyde in absolute ethanol, add pyridine dropwise under the protection of nitrogen, and heat up to 80°C to react for 7-8 hours after dropping. The resulting reaction solution was concentrated under reduced pressure to leave a residue;
[0021] ④ acidify the residue obtained in step ③ to pH 2.0 with hydrochloric acid, allow the oily product to separate out, extract with ethyl acetate, and combine the extracts;
[0022] ⑤Elute the extract obtained in step ④ with methanol and dichloromet...
Embodiment 1
[0028] Add 138g (0.87mol) of 2,3,3-trimethyl-3H-indole and 600ml of toluene into a 1000ml three-necked flask, stir until the solid dissolves, add dropwise 106g (0.87mol) of 1,3-propane sultone , the temperature was raised to 120° C. for reflux reaction for 4 hours after dropping, and the reaction was confirmed to be complete. Cool to room temperature, filter, and recrystallize the filter cake with methanol to obtain 191 g of light yellow powder (main component 2,3,3-trimethyl-1-(3-sulfonic acid propyl)-3h-indole).
[0029] In a 2000ml three-neck flask, first add 191g (0.68mol) of light yellow powder, 196g (0.52mol) of 3,5-diiodo-4-hydroxybenzaldehyde, 1500ml of absolute ethanol, stir, and add pyridine dropwise under nitrogen protection. 41g (0.52mol), after dripping, heat up to 80°C and react for 8 hours. After the reaction is over, concentrate under reduced pressure to remove the solvent. The residue is acidified to PH2.0 with 5% hydrochloric acid, and an oily product is prec...
Embodiment 2
[0031] Add 138g (0.87mol) of 2,3,3-trimethyl-3H-indole and 600ml of toluene into a 1000ml three-necked flask, stir until the solid dissolves, add dropwise 106g (0.87mol) of 1,3-propane sultone , the temperature was raised to 120° C. for reflux reaction for 4.5 hours after dropping, and the reaction was confirmed to be complete. Cool to room temperature, filter, and recrystallize the filter cake with methanol to obtain 187 g of light yellow powder (main component 2,3,3-trimethyl-1-(3-sulfonic acid propyl)-3h-indole).
[0032] In a 2000ml three-necked flask, first add 187g (0.67mol) of light yellow powder, 196g (0.52mol) of 3,5-diiodo-4-hydroxybenzaldehyde, 1500ml of absolute ethanol, stir, and add pyridine dropwise under nitrogen protection. 41g (0.52mol), after dropping, heat up to 80°C and react for 7.5 hours. After the reaction is over, concentrate under reduced pressure to remove the solvent. The residue is acidified to PH2.0 with 5% hydrochloric acid. An oily product is pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com