A kind of preparation method of lithium difluorobisoxalate phosphate, non-aqueous electrolyte and battery
A technology of lithium difluorobis oxalate phosphate and extraction liquid, which is applied to secondary batteries, chemical instruments and methods, circuits, etc. and other problems, to achieve the effect of simple steps, convenient operation, and prevention of deterioration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Take 300 mL of the reacted dimethyl carbonate mixed solution containing lithium difluorobis oxalate phosphate, and the theoretical yield of lithium difluorobis oxalate phosphate is 132 g; the mixed solution is filtered to remove insolubles, and the obtained filtrate is concentrated by distillation under reduced pressure at 50 ° C , until concentrated to an oil.
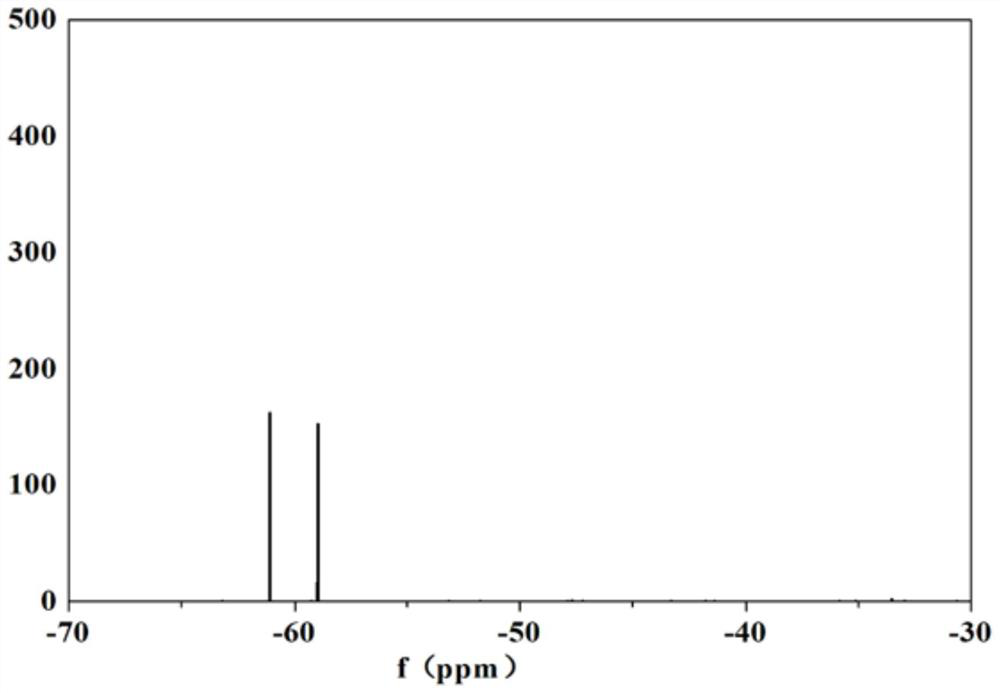
[0067] In the oil, 50 mL of methanol was added first, then 150 mL of hexane was added, and after stirring for 30 min, the solution was divided into upper and lower layers after extraction, wherein methanol was in the lower layer and hexane was in the upper layer. The methanol layer and the hexane layer were put into different beakers respectively, and the content ratio of lithium difluorophosphate and lithium difluorobisoxalate phosphate in the methanol phase was detected by nuclear magnetic fluoride spectrum was 76%: 24%; The content ratio of lithium bis-oxalate phosphate to lithium difluorophosphate is 91%: 9...
Embodiment 2
[0071] Take 500 mL of the ethyl methyl carbonate mixed solution containing lithium difluorobis oxalate phosphate after the reaction, and the theoretical yield of lithium difluorobis oxalate phosphate is 331 g; this mixed solution is filtered to remove insolubles, and the obtained filtrate is concentrated by vacuum distillation at 50 ° C , until concentrated to an oil.
[0072] In the oil, 150 mL of dimethyl sulfoxide was added first, then 600 mL of cyclohexane was added, and after stirring for 50 min, the solution was extracted into two layers, the lower layer and the upper layer were dimethyl sulfoxide. The dimethyl sulfoxide layer and the cyclohexane layer were put into different beakers respectively, and the content ratio of lithium difluorophosphate and lithium difluorobisoxalate phosphate in the dimethyl sulfoxide phase was detected by nuclear magnetic fluoride spectrum was 83%. : 17%; the content ratio of lithium difluorobisoxalate to lithium difluorophosphate in the cyc...
Embodiment 3
[0075] Take 200 mL of the propylene carbonate solution containing lithium difluorobis oxalate phosphate that has been reacted, and the theoretical yield of lithium difluorobis oxalate phosphate is 99 g; the mixed solution is filtered to remove insolubles, and the obtained filtrate is concentrated under reduced pressure distillation at 50 ° C until Concentrated to an oil.
[0076] In the oil, 40 mL of dimethylformamide was added first, followed by 150 mL of heptane, and after stirring for 60 min, the solution was extracted into upper and lower layers, wherein dimethylformamide was in the lower layer and heptane was in the upper layer. The dimethylformamide layer and the heptane layer were put into different beakers, and the content ratio of lithium difluorophosphate and lithium difluorobisoxalate phosphate in the dimethylformamide phase was 69%: 31% after nuclear magnetic detection. ; The content ratio of lithium difluorobisoxalate to lithium difluorophosphate in the heptane ph...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com