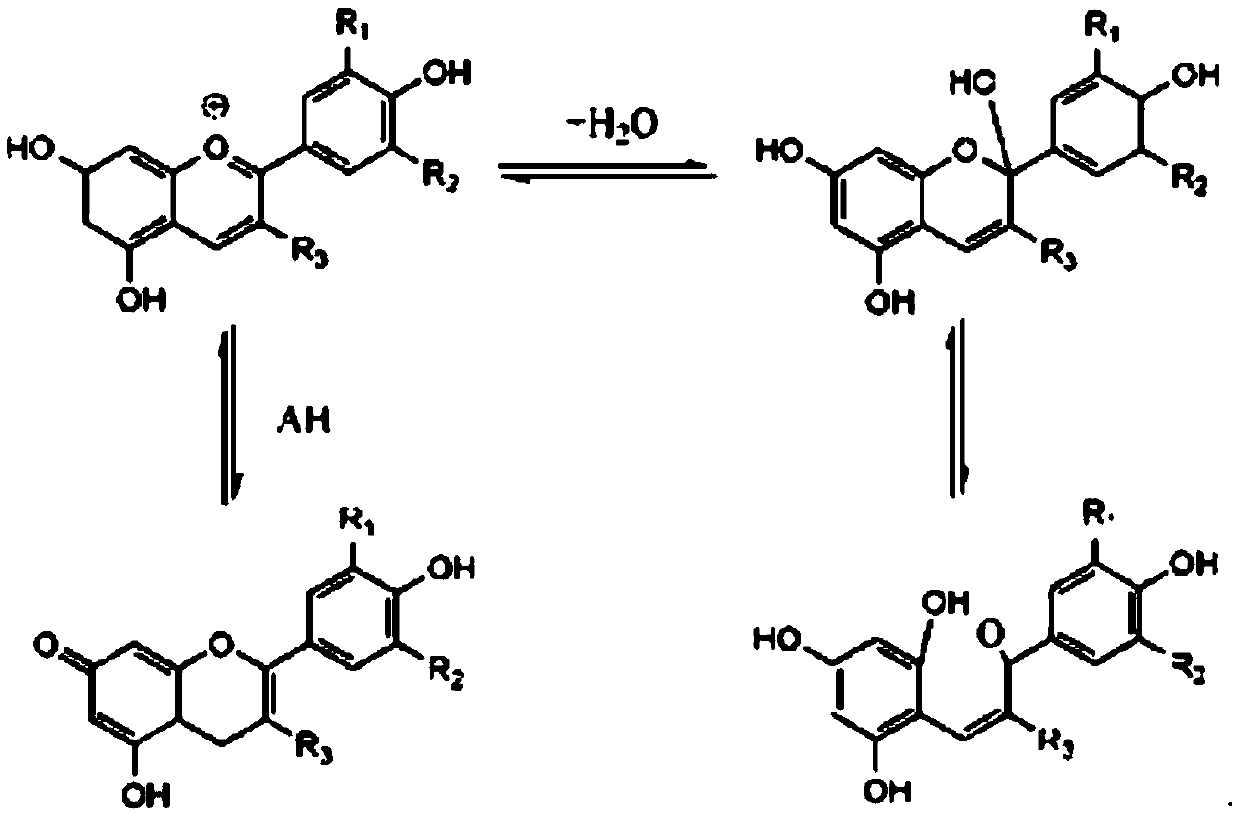
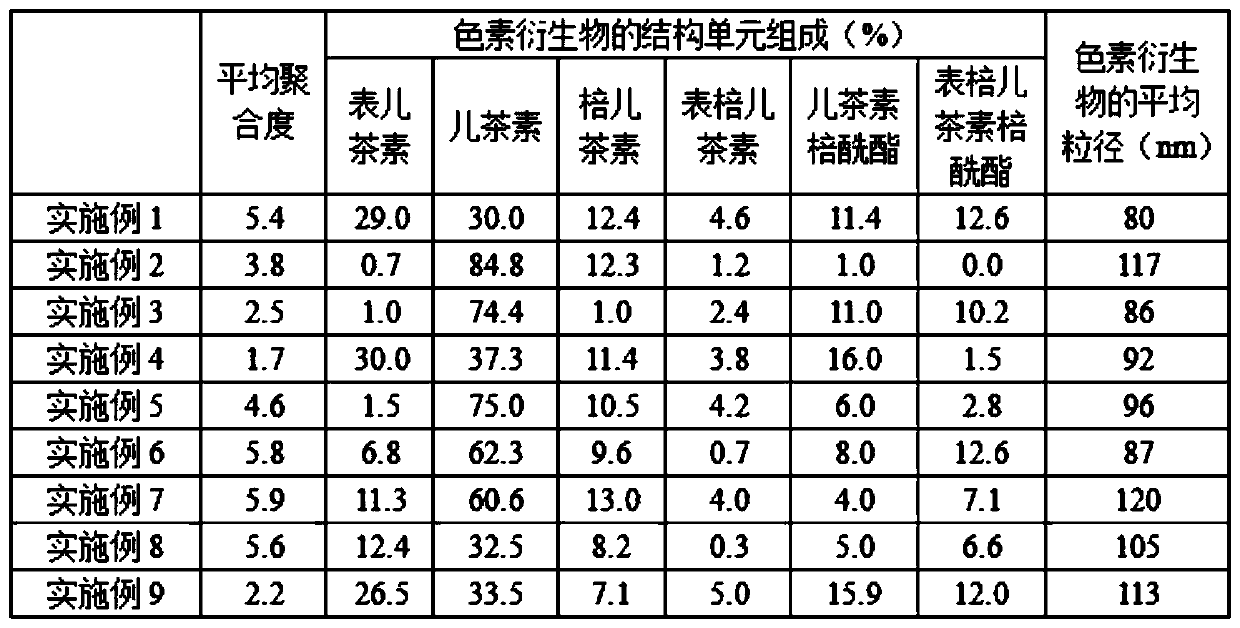
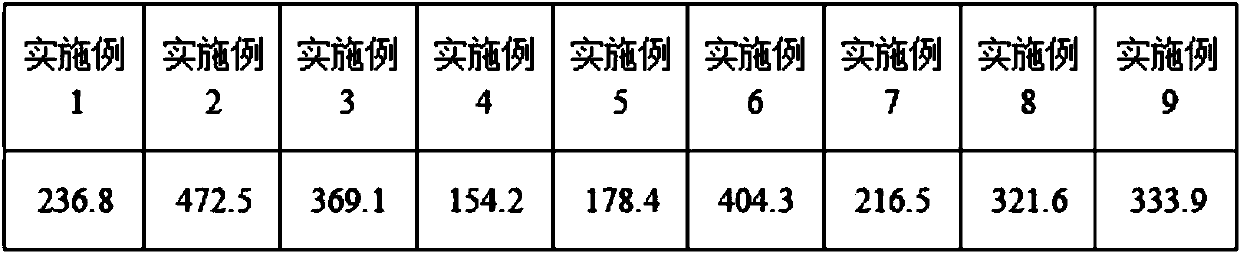
High-stability pigment derivative and preparation method thereof
A pigment derivative and high-stability technology, applied in the field of pigments, can solve problems such as sensory quality decline, decrease, and balance decrease, and achieve the effects of increasing production efficiency, wide source of raw materials, and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]Add 2 parts of mallow pigment-3-glucoside into the reactor, add 1000 parts of distilled water, stir for 25 minutes, then add 1.2 parts of proanthocyanidins with an average degree of polymerization of 1.5, stir fully for 1 hour, add 1.5 parts of acetaldehyde diethyl acetal, stirred for 25 minutes, after the mixing process, add 0.2 parts of cinnamic acid, at this time the pH of the system is 5.6, the reactor is sealed and protected from light, and placed in an environment with a temperature of 20°C for 120 days , and then the finished product is obtained after opening the reactor for drying.
Embodiment 2
[0026] Add 15 parts of mallow pigment-3-glucoside into the reactor, add 200 parts of distilled water, stir for 25 minutes, then add 60 parts of proanthocyanidins with an average degree of polymerization of 2.7, stir fully for 1 hour, add 10 parts of propionaldehyde, stirred for 25 minutes, after the mixing process, add 0.4 parts of tartaric acid, at this time the pH of the system is 1.2, the reactor is sealed and protected from light, and placed in an environment with a temperature of 35°C for 270 days, and then the reactor is opened The finished product is obtained after drying.
Embodiment 3
[0028] Add 10 parts of geranium pigment-3-glucoside into the reactor, add 5000 parts of distilled water, stir for 25 minutes, then add 90 parts of proanthocyanidins with an average degree of polymerization of 3.8, stir fully for 1 hour, add B 12 parts of aldehyde, stirred for 25 minutes, after the mixing treatment, add 0.2 parts of hexanoic acid, at this time the pH of the system is 1.2, seal the reactor to avoid light, place it in an environment with a temperature of 25°C for 5 days, then open the reactor The finished product is obtained after drying.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com