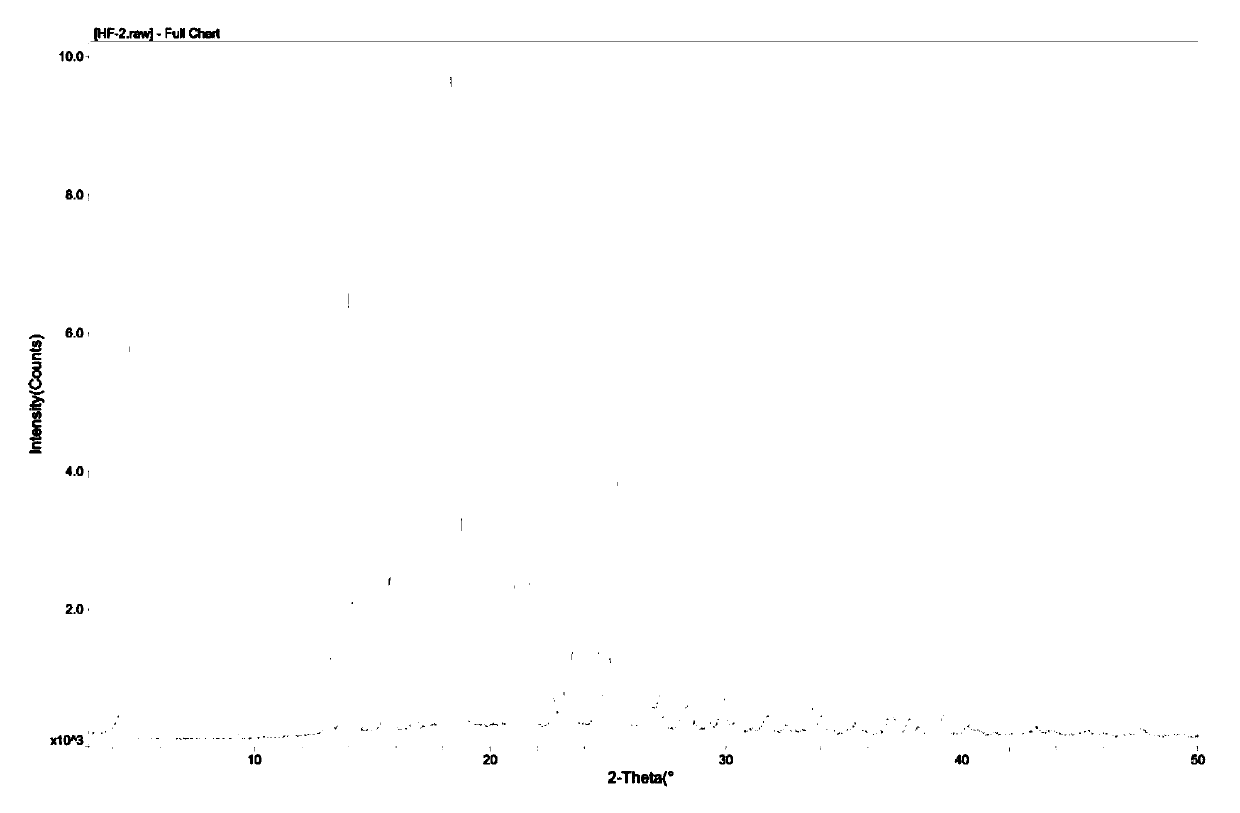
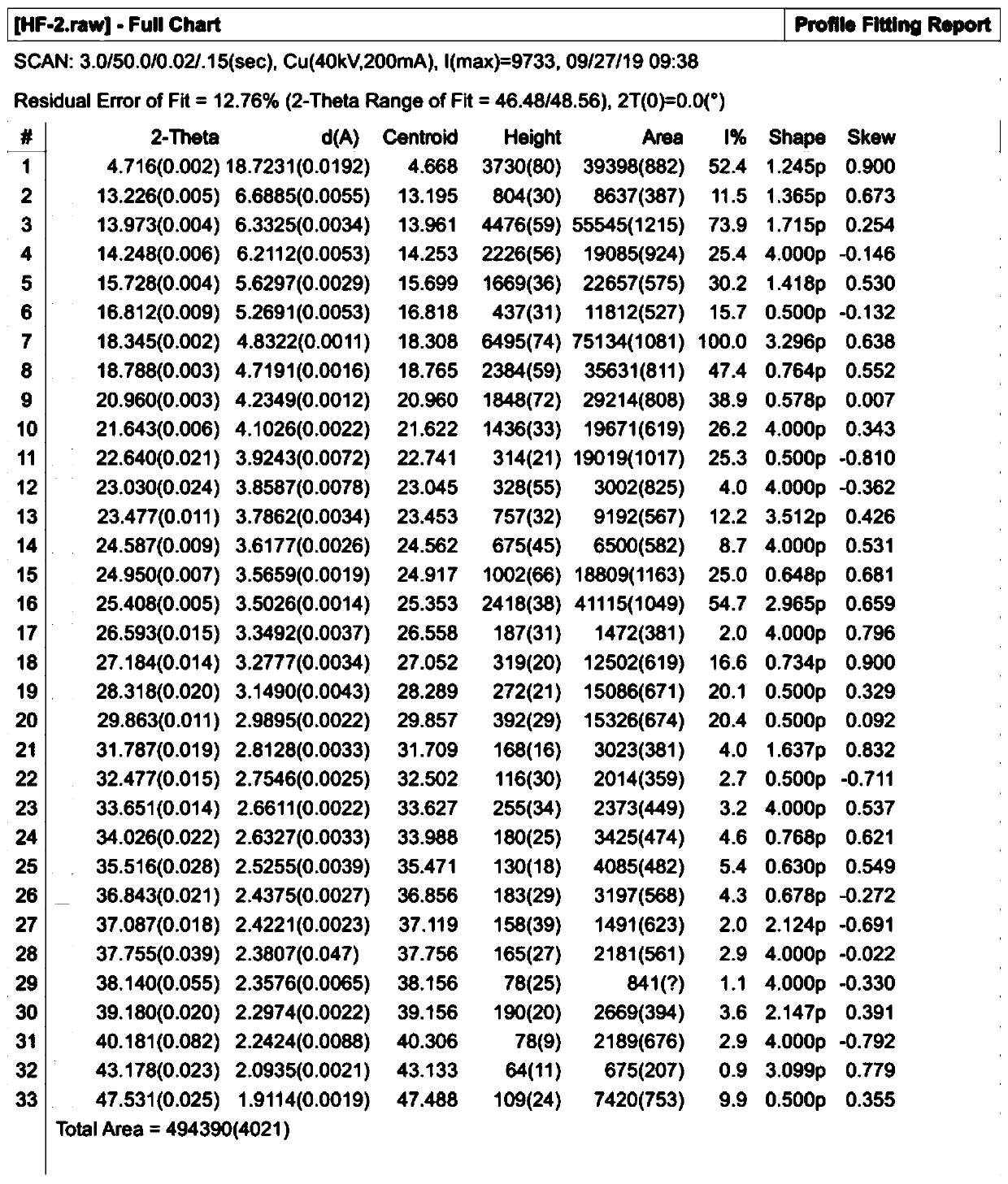
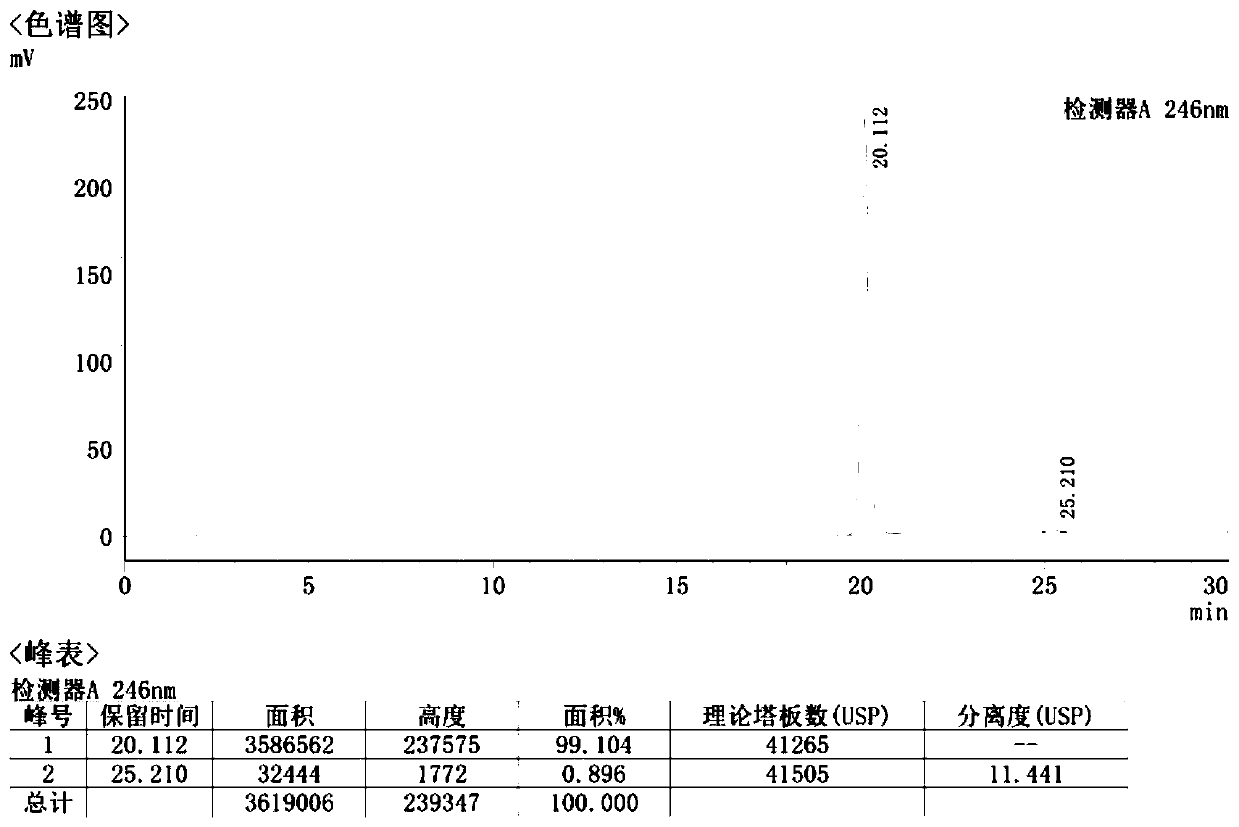
Propacetamol crystal form and preparation method thereof
A technology of propatamole and crystal form, applied in the field of chemical organic synthesis, can solve the problems of increased cost, difficulty in the purification of the next step, unqualified detection items such as clarity and color, etc., so as to prevent excessive or excessive use of hydrochloric acid. less effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add 100.0g (0.6615mol, 1eq) of acetaminophen, 1L of acetone, and 110.4g (0.8000mol, 1.2eq) of anhydrous potassium carbonate into a three-necked flask, mechanically stir and slowly add chlorine in an ice bath at 0-5°C Acetyl chloride 63mL (0.8000mol, 1.2eq), continue to react under ice bath for 0.5h after dropping, and react at room temperature for 6h. After the reaction was completed, 5.5 g (0.0662 mol, 0.1 eq) of potassium iodide was added to the reaction solution, and 105.5 g (1.4424 mol, 2.18 eq) of diethylamine was added at 40° C., reacted for 2 hours, filtered, and 5 g of activated carbon was added to the filtrate, refluxed for decolorization, and filtered. Evaporated to dryness under reduced pressure, dissolved in 200 mL of ethyl acetate, slowly added dropwise 600 mL of n-heptane to crystallize, filtered, and dried under reduced pressure at 30°C to obtain 119.8 g of off-white solid with a yield of 68.5% and a purity of 99.1%.
Embodiment 2
[0034] Add 100.0g (0.66mol, 1eq) of paracetamol, 1L of acetone, and 63.3g (0.80mol, 1.2eq) of pyridine into a three-neck flask, mechanically stir and slowly add 63mL of chloroacetyl chloride in an ice bath at 0-5°C (0.80mol, 1.2eq), continue to react in ice bath for 0.5h after dropping, and react at room temperature for 6h. After the reaction was completed, 5.5 g (0.07 mol, 0.1 eq) of potassium iodide was added to the reaction solution, and 105.5 g (1.44 mol, 2.18 eq) of diethylamine was added at 40° C., reacted for 2 hours, filtered, and 5 g of activated carbon was added to the filtrate, refluxed for decolorization, and filtered. Evaporate to dryness under reduced pressure, dissolve in 200 mL of ethyl acetate, slowly add 600 mL of n-heptane to crystallize, filter, and dry under reduced pressure at 30°C to obtain 105.8 g of off-white solid with a yield of 60.5% and a purity of 98.1%.
Embodiment 3
[0036] Add 100.0g (0.66mol, 1eq) of acetaminophen, 1L of acetone, and 110.4g (0.80mol, 1.2eq) of anhydrous potassium carbonate into a three-neck flask, mechanically stir and slowly add chlorine dropwise under an ice bath at 0-5°C Acetyl chloride 69mL (1.00mol, 1.5eq), continue to react in ice bath for 0.5h after dropping, and react at room temperature for 6h. After the reaction was completed, 5.5 g (0.07 mol, 0.1 eq) of potassium iodide was added to the reaction solution, and 105.5 g (1.44 mol, 2.18 eq) of diethylamine was added at 40° C., reacted for 2 h, filtered, and 7 g of activated carbon was added to the filtrate, refluxed for decolorization, and filtered. Evaporated to dryness under reduced pressure, dissolved in 200 mL of ethyl acetate, slowly added dropwise 600 mL of n-hexane to crystallize, filtered, and dried under reduced pressure at 30°C to obtain 121.4 g of off-white solid with a yield of 69.4% and a purity of 98.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com